PISUM GENETICS
2011-VOLUME 43
RESEARCH PAPERS
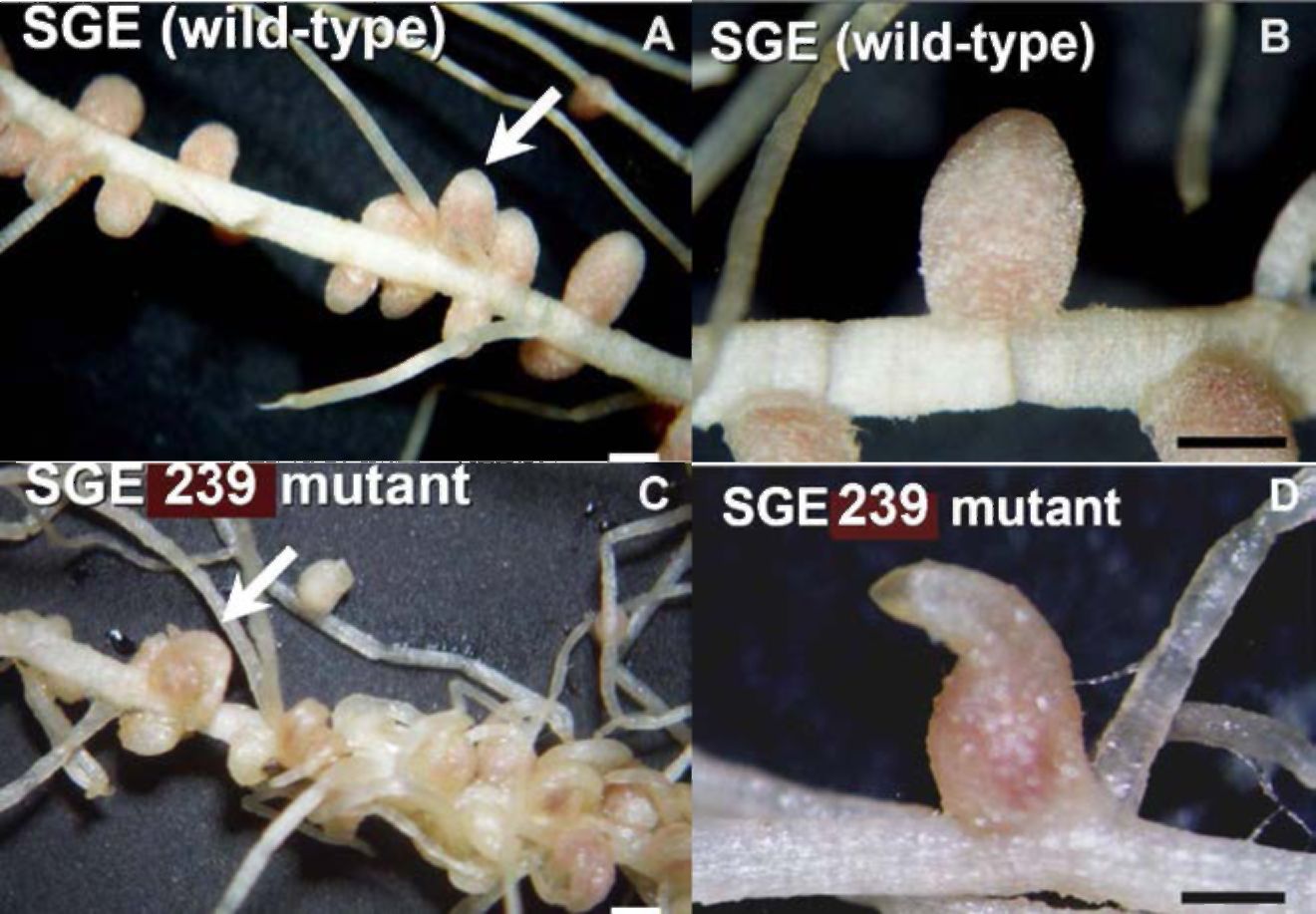
Figure 1. The root nitrogen-fixing nodules of mutant coch line SGE239 (C,D) and SGE ancestor line (A, B).
The stage at which nodules in the SGE239 mutant were transformed to a rootlet was identified by
microscopic analysis. It was found that early stages of root nodule development in the mutant were
similar to the parental line SGE.
Both wild type and mutant plants show the normal process of root hair curling, infection thread growth,
nodule induction and primordium formation. Then similarly in both lines the infection thread penetrates
nodule primordium, bacteria endocytosed into plant cell cytoplasm and immature nodular circular
meristem is differentiated at the distal end of the nodule primordium. The meristem of mature
indeterminate nodules is formed from one part of the circular meristem at the next stage of wild-type
nodule development. But at the same time in SGE239 nodules a root meristem is developed from the part
of nodular immature circle meristem at the distal end of young nodules. At a later stage, root growth in
the mutant is observed at the tip of the nodule and the vascular system of this rootlet is connected to one
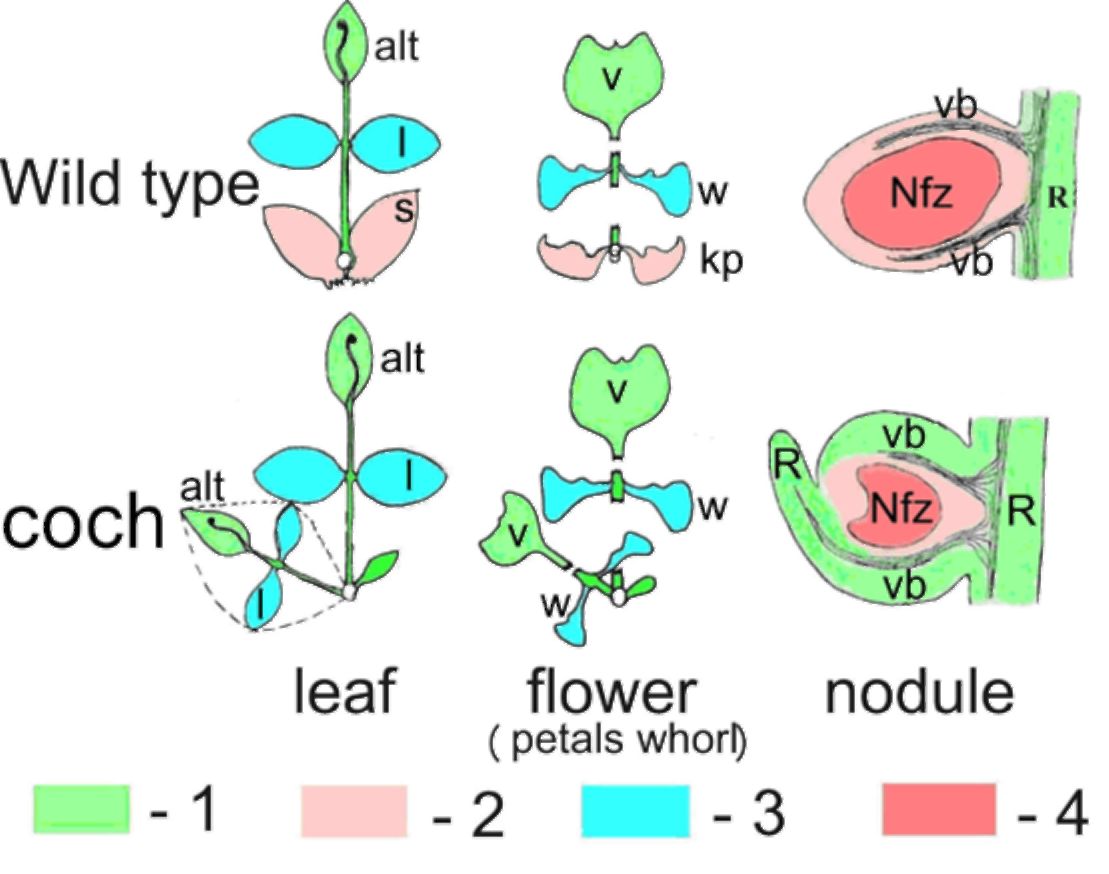
of the vascular bundles of the nodule (Fig. 2). Thus, the pea gene Coch acts at the stage of mature
indeterminate nodule meristem development, specifying nodule or root meristem identity.
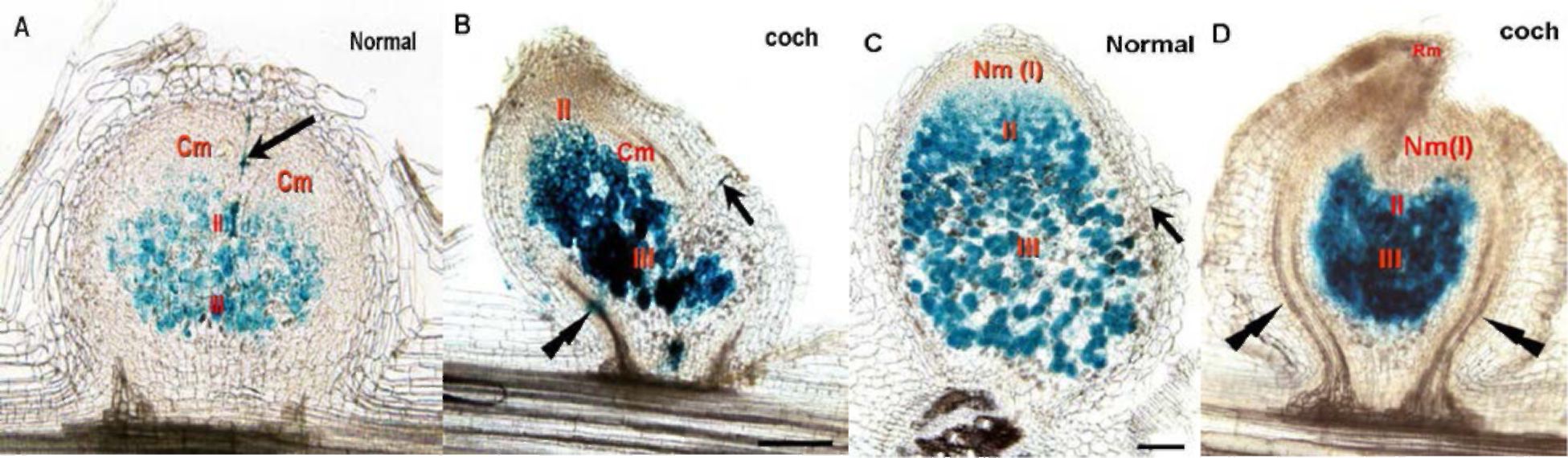
Figure 2. Two late stages ofpeea root nodule formation. A,C — ancestor line SGE; B,D — coch mutant line SGE239. Arrows indicate the infection threads; Double arrows specify the formingroot vascular system. Cm — immature circular nodular meristem; Nm (I) — mature nodular meristem; II—infection (endocytosis) zone; III—nitrogen-fixation zone; Rm — rootlet meristem.
7