Pisum Genetics
2010-Volume 42
Research Papers
On intraspecific variation of Vavilovia formosa (Stev.) Fed. (= Pisum formosum (Stev.) Alef.: Fabeae)
Sinjushin, A.A. and M.V. Lomonosov Moscow State University, Moscow, Russia
Belyakova, A.S.
The phylogeny and systematics of tribe Fabeae Rchb. are still somewhat unclear. One of the most enigmatic genera within this group is Vavilovia Fed. representing small highland plants with very specific features. It inhabits disrupted areas in Caucasus and Middle East and is commonly referred to as an endangered plant species. Numerous research reports dealing with its morphology, anatomy and taxonomical position (evidenced from results of both classical and molecular analyses) exist (1, 6 and works cited in reviews of these papers). Expressed interest in this genus is evidenced by the appearance of several publications during the last few years (7, 8, 9 etc.). Exhaustive surveys on the history of investigations on this plant species have recently been published (6, 7).
Since this genus was described by C. Steven in 1812 (see 7 for details), numerous data on its intrageneric differentiation were reported. Table 1 summarizes the history of microsystematics of Vavilovia with different synonyms listed. The present work together with (11) represent efforts to reveal any intrageneric variation within Vavilovia (regardless of whether we treat it as a Pisum species or as separate genus).
Table 1 Correspondence between different synonyms of intraspecific taxa within Vavilovia.
|
Source
|
|
|
||
|
(4)
|
Pisum formosum var. typicum Gov.
|
P. formosum var. microphyllum Ser.
|
||
|
(2)
|
Vaviloviaformosa (Stev.) Fed.
|
V. aucheri Fed.
|
||
|
(3)
|
V.formosa (Stev.) Fed.
|
|||
|
(5)
|
Alophotropis formosa (Stev.) A. Grossh. | A. aucheri (Jaub. & Spach) A. Grossh.
|
|||
Materials and Methods
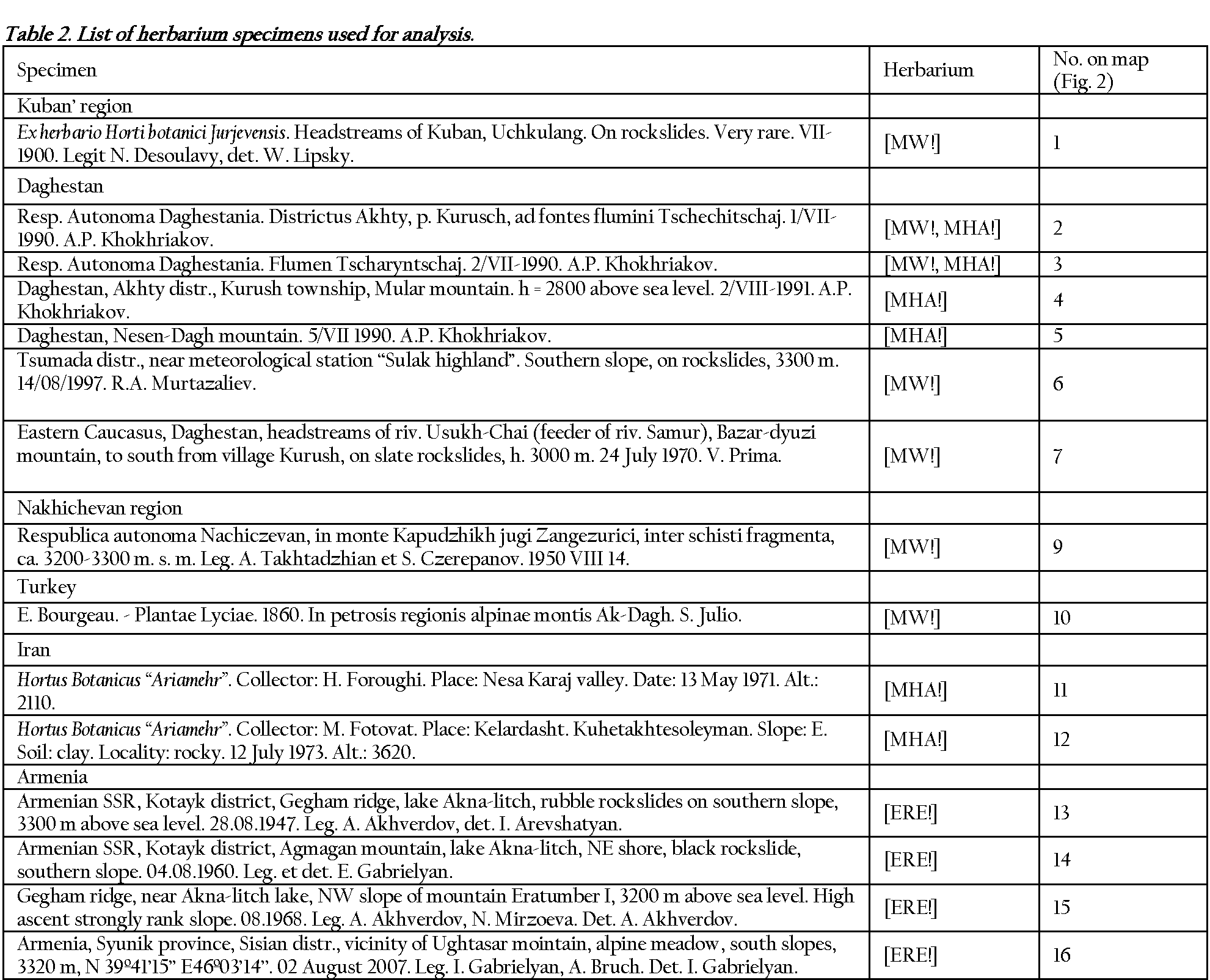
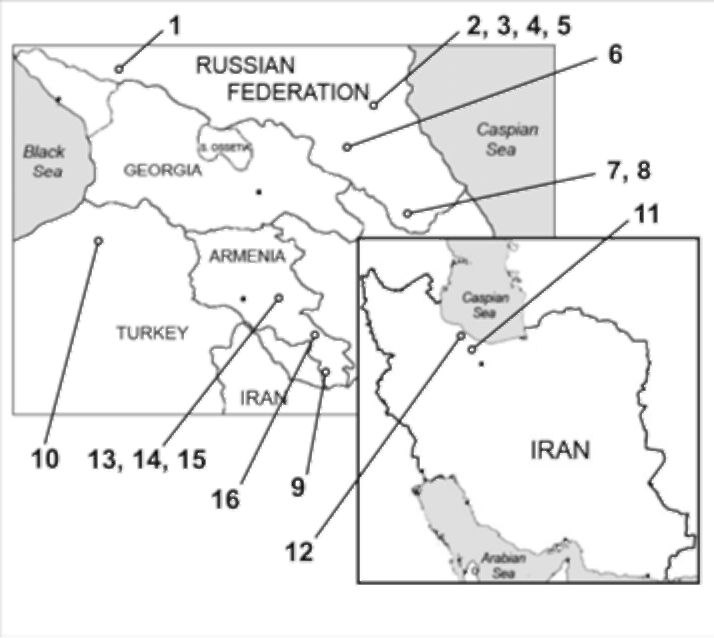
The morphometric analysis was performed using photo images of herbarium specimens (MW!, MHA!, ERE!, WIR!). In total 22 specimens were analyzed and 412 leaflets measured. The chosen samples
Figure 1 Map depicting collection sites of herbarium samples. Numbers correspond to those in Table 2.
represented collections of different years (1860-2007) from territories of the Kuban' region, Daghestan, Armenia, Turkey
and Iran; for details see Figure 1 and Table 2.
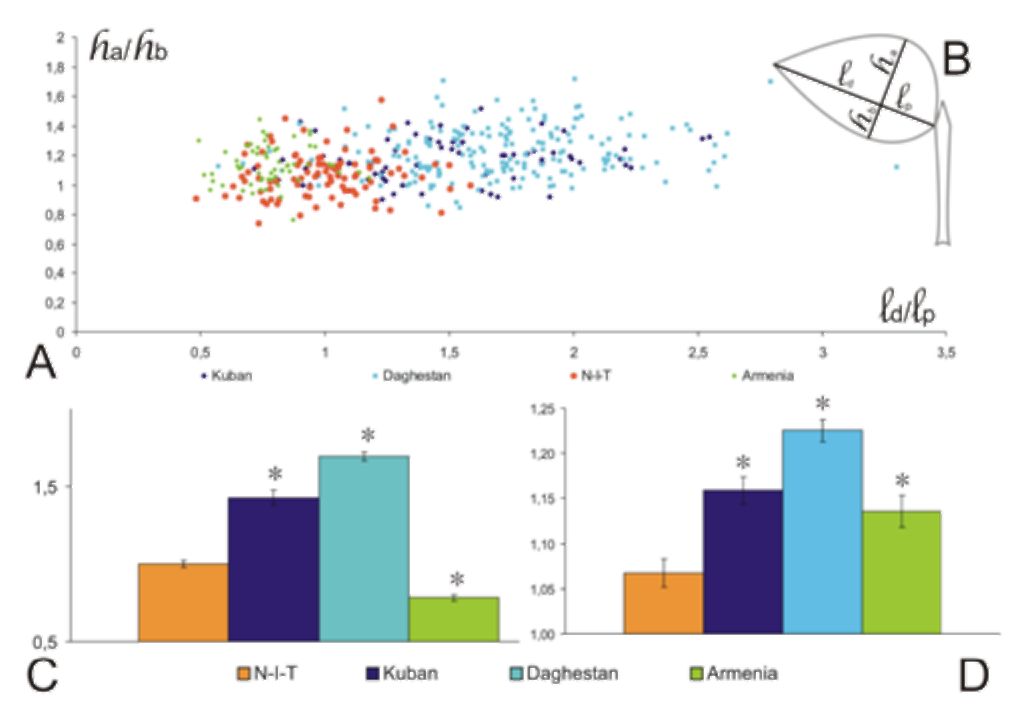
All samples were photographed or scanned. The leaves were measured with reference to two parameters, longitudinal and bilateral asymmetry of the leaflet, viz. distance between leaflet base and position of maximum width (lp), distance between leaflet tip and position of maximum width (ld), maximum distances from midvein to leaf margin from basiscopic (hb) and acroscopic (ha) sides (Fig. 3B). All measurements were performed using Meazure™ 2.0 program (C Thing Software). The obtained data were analyzed with the Statistica 8 (Statsoft Inc., Tulsa, OK, USA) software package.
31