Pisum Genetics
2010-Volume 42
Research Papers
Contributions to the characterization of Vavilovia formosa (syn. Pisum formosum). II. Morphology of androecium and gynoecium and mitosis
Atlagic, J.1, Mikic, A.1, institute of Field and Vegetable Crops, Novi Sad, Serbia
Sarukhanyan, N.2, Vanyan, A.2, 2Green Lane Agricultural Assistance NGO, Yerevan, Armenia
Akopian, J.3, Gabrielyan, I.3, 3National Academy of Sciences, Institute of Botany, Yerevan, Armenia
Smykal, P.4, Kenicer, G.5, 4Agritec Plant Research Ltd., Sumperk, Czech Republic
Vishnyakova, M.6 and 5Royal Botanical Garden Edinburgh, Edinburgh, UK
Ambrose, M.7 6N. I. Vavilov Institute of Plant Industry, St. Petersburg, Russia
7John Innes Centre, Norwich, UK
Vavilovia (Vavilovia formosa Stev. Fed., syn. Pisum formosum) is the only species of the genus Vavilovia Fed. included in the tribe Fabeae with vetchling (Lathyrus L.), lentil (Lens Mill.), pea (Pisum L.), and vetch (Vicia L.) (1). Largely ignored for decades, V. formosa has become the object of recent attention by geneticists and molecular taxonomists (2) due to its specific position within the tribe and the supposed role in its evolution (3).
The flowers of V. formosa are often solitary, axillary and pedunculate, with small and/or inconspicuous bracts, lacking bracteoles, and having a campanulate calyx. The corolla of V. formosa is pink or purple with an oblong standard, falcate to oblong wing and blant, non-cristate and sometimes white keel (4).
This preliminary research was aimed at examining the morphology of the reproductive organs of V. formosa with emphasis on the androecium and gynoecium, as well as understand some basic cytogenetics of the species.
Materials and Methods
The first of the three expeditions aimed at in situ research and ex situ conservation of V. formosa in Armenia was carried out to the Mount Ughtasar in southern Armenia, on July 17, 2009 (5). During this expedition, samples of stems, leaves and flowers of one population growing at an altitude of between 3305 and 3315 masl were collected and fixed in Carnoy I, a 3:1 solution of ethanol and glacial acetic acid.
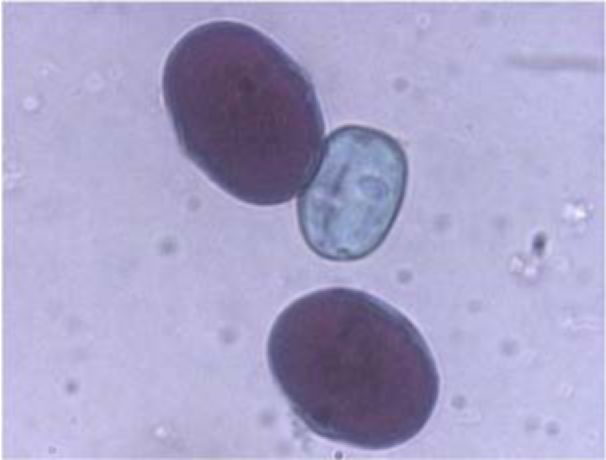
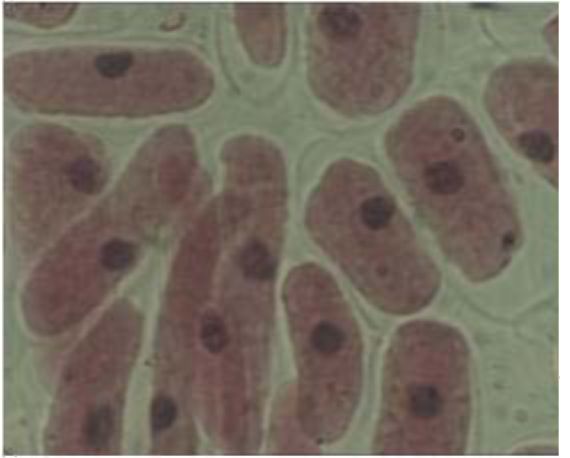
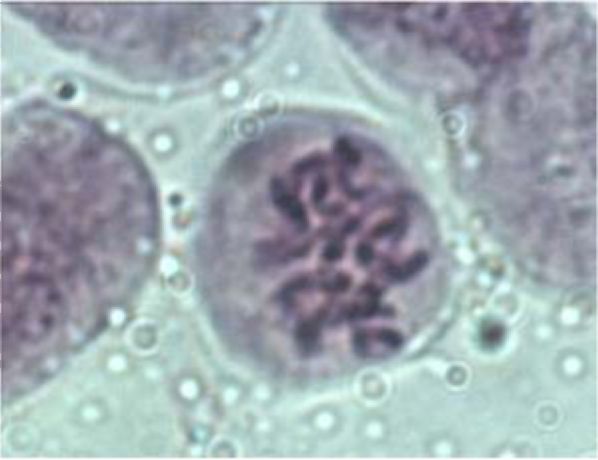
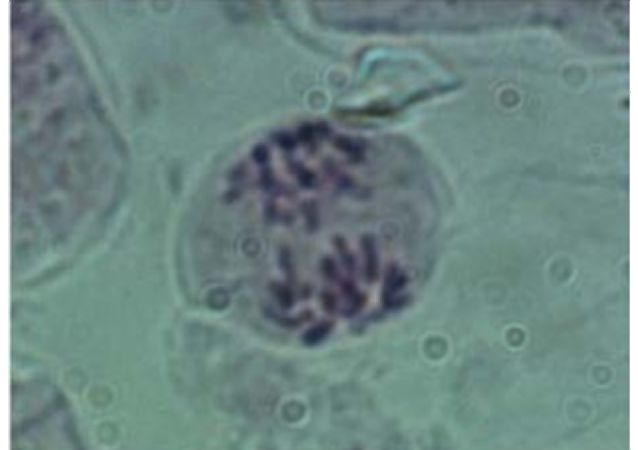
The parts of V. formosa flowers were observed using stereo microscope Stereo microscope Stemi 2000 (Carl Zeiss, Gottingen, Germany) and photographed by the Power Shot G5 Digital Camera (Canon, Japan). The pollen grains were removed from the anthers and their viability was determined using the staining method of Alexander (6). The youngest leaves from the fixed material were used to determine the chromosome number using the acetocarmine method (7).
Results and Discussion
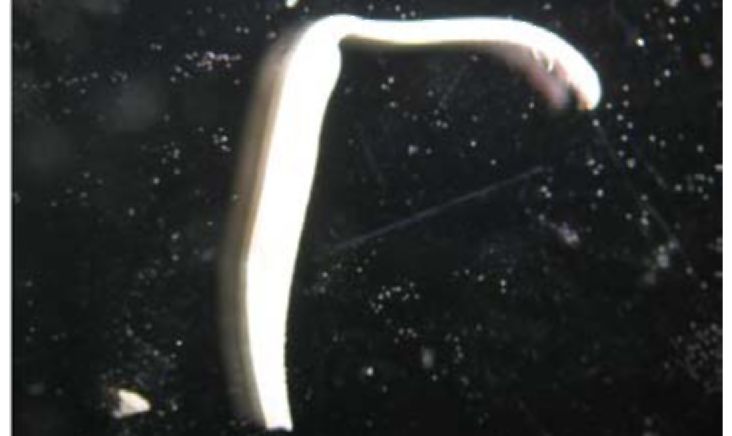
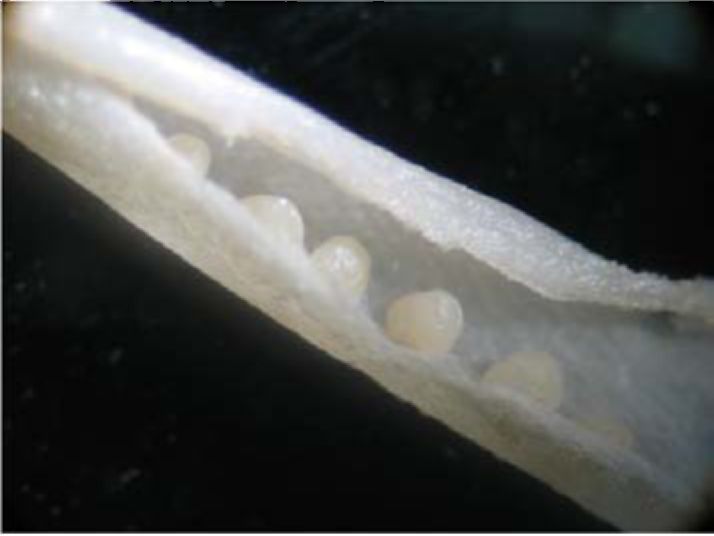
Androecium. As in many other legume species, the androecium in V. formosa is diadelphous, where nine filaments are fused together and anthers are spherical and two-pieced (Fig. 1). In some fully developed flowers only one or two filaments had anthers. In the case of fully developed flowers with regular
Figure I. Stamens of V. formosa en velopingpistil (lower right comer).

25