Y15_999Fw, a dominant SCAR marker
linked to
the Fusarium wilt race 1 (Fw)
resistance gene in pea
Okubara,
P.A.1, 1USDA-ARS Root Disease and Biol. Control,
Keller,
K.E. 2, 2USDA-ARS Hort. Crops Res. Lab.,
McClendon,
M.T. 3,
3Dept. of Crop and Soil Sci.,
Inglis,
D.A. 4, 4Northwestern
McPhee,
K.E. 5 and
Washington State Univ.,
Coyne,
C.J. 6 5
USDA-ARS Grain Legume Genet. and
6USDA-ARS,
WRPIS,
Fusarium
wilt, caused by Fusarium oxysporum Schlecht. emend. f. sp. pisi
(van Hall) Snyd. & Hans., poses significant yield losses to Pisum sativum
L. (green pea) in the
The objective of this study was to develop a sequence characterized amplified region (SCAR) marker for Fw to be used in marker assisted selection (MAS). If closely linked to a trait of interest, such a molecular marker provides a high probability that the trait is present. Use of molecular markers can conserve resources if the desired trait is exhibited late in development (requires time), or scoring of the phenotype is complicated or requires a high degree of expertise. McClendon et al. (9) identified three dominant markers for Fw, two amplified fragment length polymorphism (AFLP) markers ACC:CTG_159 and ACG:CAT_222 that flank Fw over a 4.0 cM interval, and a random amplified polymorphic DNA (RAPD) marker Y15_1050 that lies approximately 4.6 cM upstream of Fw. Here, we report the nucleotide sequence of RAPD-derived Y15_1050 PCR product, the development of a 999 bp SCAR marker, and the identification of a Y15 allele linked in coupling to susceptiblity.
Materials and Methods
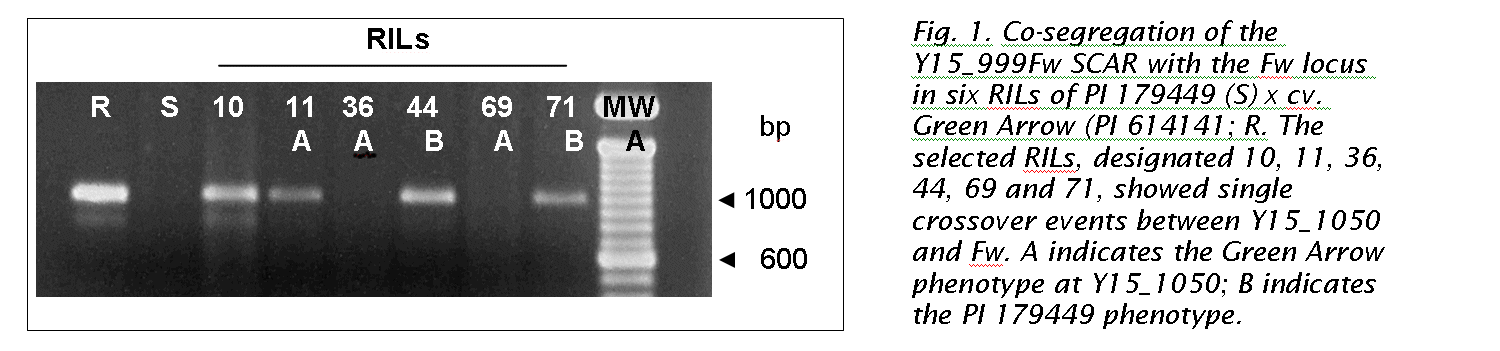
Plant materials and DNA were generated as previously described (9). Eighty F8-derived recombinant inbred lines (RIL) from a cross of PI 179449 (susceptible) and cv. Green Arrow (PI 614141, resistant) were tested for resistance to Fusarium wilt race 1 in two greenhouse trials and one field test. The RIL mapping population contained six individuals that showed single recombination events between Y15_999Fw and the Fw locus (Table 2); four recombinants displayed the Green Arrow phenotype at Y15_999Fw (PRIL 7-10, PRIL 7-11, PRIL 7-44, PRIL 7-71) and two displayed the susceptible (null) phenotype (PRIL 7-36 and PRIL 7-69). Genomic DNA was prepared from young leaf tissue of the parents and RILs using a modification (16) of the DNA extraction method of Murray and Thompson (11). DNA concentration was estimated by comparing ethidium bromide staining intensity to that of DNA ladder standards of known concentrations. Leaf DNA concentration was adjusted to 25 ng/μl, then screened using RAPD oligonucleotides (OPERON Technologies, Alameda, CA) as described by Paran and Michelmore (13).
Primer Y15 (Table 1) amplified a ~1050 bp product that co-segregated with Fw (7) in the resistant parent Green Arrow, but not in the susceptible parent, PI 179449. To clone the Y15_1050 PCR amplicon, the RAPD
Table 1. RAPD and SCAR primers used in this study. All primers are shown in the 5’ to 3’ orientation.
|
Amplicon |
Assay |
Forward primer |
Reverse primer |
Positiona |
Tm (oC) |
Product (bp) |
|
Y15_1050 |
RAPD |
5’-AGTCGCCCTT-3’ |
same |
1 |
36 |
1082 |
|
Y15_999Fw |
SCAR |
5’-ATGAGGGTAGC GCTTCATTG-3’ |
5’-GCCCTTTGTTG TCTCACCTG-3’ |
81 |
60 |
999 |
a Starting nucleotide position of the forward primer on the Y15_1050 sequence (GenBank DQ189096).
Table 2. RILs showing recombination between Fusarium wilt race 1 resistance (Fw) and two markers, RAPD Y15_1050 and SCAR Y15_999Fw, in the PI 179449 (susceptible) x Green Arrow (resistant) F8 RIL mapping population. A, Green Arrow parental type; B, PI 179449 parental type.
|
Line |
Type |
Fw phenotypea |
Y15_1050 |
Y15_999Fw |
|
Green Arrow |
R parent |
A |
A |
A |
|
PI 179449 |
S parent |
B |
B |
B |
|
PRIL7-10 |
RIL |
B |
A |
A |
|
PRIL7-11 |
RIL |
B |
A |
A |
|
PRIL7-36 |
RIL |
A |
B |
B |
|
PRIL7-44 |
RIL |
B |
A |
A |
|
PRIL7-69 |
RIL |
A |
B |
B |
|
PRIL7-71 |
RIL |
B |
A |
A |
aScoring based on data from two greenhouse trials conducted in 1999 and 2000, and one replicated field trial (three plots) conducted in 2000.
PCR reaction (9) was run at an annealing
temperature of 36 C. The PCR product was separated on a 1% agarose gel (low OEO,
Fisher Scientific,
Two sets of SCAR primers (Table 1) were designed using Primer3 software
at the default parameters (14). The SCAR assays (25 μl) consisted of 1 unit
of Taq polymerase in 1X buffer (Roche Applied Science,
The Y15_1050 nucleotide sequence was analyzed for open reading frames using MapDraw and the Blastx algorithm (1) of GenBank. The Medicago truncatula gene index of TIGR (www.tigr.org/tdb/tgi) and GenBank were searched for Y15_1050 homologs using Blastn (1).
To increase its utility in MAS, the Y15_1050 RAPD marker from the Fw pea cultivar Green Arrow was cloned, sequenced and used to develop SCAR primers. Three of four Y15 inserts were of the expected size of ~1050 bp and had an identical 1082 bp nucleotide sequence (GenBank accession no. DQ189096), whereas the fourth insert was approximately 1000 bp.
Initially, two SCAR primer pairs (Table 1) were designed from the 1082 bp Y15_1050 sequence and tested on the parents and RIL mapping population. One of the primer pairs amplified a 999 bp fragment, designated Y15_999Fw (Fig. 1, Table 1), from Green Arrow and 38 RILs that displayed the Green Arrow genotype at Y15_1050 (data not shown). All 37 RILs that showed the PI 179449 (susceptible) genotype at Y15_1050 were negative for the 999 bp SCAR amplicon. In addition, these SCAR primers resolved ambiguous RAPD scores in three other RILs (data not shown). In initial screens, two RILs displayed discrepancies between the RAPD and SCAR results. The RILs shown in Fig. 1, exhibiting single crossover events between Y15_1050 and Fw, showed the expected Y15_999Fw SCAR genotype. In short, the Y15_999Fw SCAR marker remained polymorphic (dominant) and reliably reflected the presence of the original RAPD marker in the parental lines and the RIL mapping population. The second SCAR primer pair generated a 201 bp amplicon that was monomorphic in both resistant and susceptible parents, and from the six RILs in the Y15_1050 - Fw interval (data not shown).

The absence of long open reading frames within the 1082 bp Y15 RAPD amplicon, the lack of significant matches to proteins in GenBank, and the dominant nature of the Y15_999Fw SCAR indicated that this molecular marker was not derived from an open reading frame. However, two segments of the Y15_1050 sequence totaling 150 bp showed an average of 86% nucleotide sequence identity (E value 4e-09) to the non-coding region of a mariner-like transposable element from P. sativum (GenBank AY833550). Mariner-like elements have been observed in a wide range of eukaryotes (2), including a member of the family Fabaceae (5), and the detection of transposons by RAPD primers is not surprising. More genomic sequence data will be needed to determine whether the Y15_1050 and Y15_201Fw amplicons are located in mariner-like elements in Green Arrow and PI 179449.
The Y15_999Fw SCAR will provide breeders with a robust, dominant molecular marker with which to follow the Fw locus in approximately 95% of the progeny. This marker is one of several that can be used in MAS and in further characterization of the Fw locus. The AFLP markers ACC:CTG_159 and ACG:CAT_222 that flank Fw are being sequenced, and segregation analyses using these markers is in progress. Additional markers in pea, including resistance gene analogs (17) and microsatellites (8), can also be adapted for MAS.
Inverse PCR and primer walking strategies will be undertaken to obtain additional sequence information for developing a co-dominant Y15 marker. Such a marker could be primer-based (e.g., SCAR), a RFLP, or a single nucleotide or conformational polymorphism. For example, insertions and deletions are observed at the Y15_999Fw locus in two of 96 individuals sampled from the Pisum core collection (15) (Coyne and Timmerman-Vaughn, unpublished data). A dramatic migration shift between the one of these variants and the parental amplicon was observed in a SSCP gel (Coyne and Timmerman-Vaughan, unpublished data), making this type of marker highly scorable. These observations indicate several approaches for the development of a co-dominant Y15 marker that could be used to distinguish Fw homozygotes from heterozygotes in breeding programs.
Acknowledgments:
The authors thank Weidong Chen and Shanmukh Salimath for critical reading of the
manuscript. This work was supported by ARS Project Nos. 5348-21000-017-00D (CJC)
and 5248-22000-012D (PAO), and a grant from the USDA-CSREES Cool Season Food
Legume Special Grant Program (DAI, KEM, CJC, MTM). Nucleotide sequencing of
Y15_201Fw was done at the Laboratory for Biotechnology and
1. Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Nucleic Acids Res. 25: 3389-3402.
2. Bigot, Y., Brillet, B. and Augé-Gouillou, C. 2005. J. Mol. Biol. 351: 108-116.
3. Grajal-Martin, M. J. and Muehlbauer, F.J. 2002. J. Hered. 93: 291-293.
4.
Haglund, W.A. and Pepin, H.S. 1987.
5. Jarvik, T. and Lark, K.G. 1998. Genetics 149: 1569-1574.
6. Kraft,
J.M. and Pfleger, F.L. 2001. Compendium
of Pea Diseases, 2nd ed., American Phytopathological Society Press,
7. Laucou, V., Haurogne, K., Ellis, N. and Rameau, C. 1998. Theor. Appl. Genet. 97: 905–915.
8. Loridon, K., McPhee, K.E., Morin, J., Dubreuil, P., Pilet-Nayel, M.L., Aubert, G., Rameau, C., Baranger, A., Coyne, C.J., Lejeune-Hènault, I. and Burstin, J. 2005. Theor. Appl. Genet. (DOI 10.1007/s00122-005-0014-3).
9. McClendon, M.T., Inglis, D.A., McPhee, K.E. and Coyne, C.J. 2002. J. Amer. Soc. Hort. Sci. 127: 602-607.
10. McPhee, K.E., Tullu, A., Kraft, J.M. and Muehlbauer, F.J. 1999. J. Amer. Soc. Hort. Sci. 124: 28-31.
11.
12. Nicholas, K.B. and Nicholas, H.B., Jr. 1997. www.psc.edu/biomed/genedoc.
13.
Paran,
14.
Rozen, S. and Skaletsky, H.J. 2000.
In: Krawetz S, Misener S (eds.) Bioinformatics
Methods and Protocols: Methods in Molecular Biology. Humana Press,
15. Simon, C.J. and Hannan, R.J. 1995. HortSci. 30: 907.
16. Simon, C.J. and Muehlbauer, F.J. 1997. J. Hered. 88: 115-119.
17. Timmerman-Vaughan, G.M., Frew, T.J. and Weeden, N.F. 2000. Theor. Appl. Genet. 101: 241-247.