ITS
sequence variation in wild species and cultivars
of pea
Polans,
N.O. and Saar, D.E.
Dept. of Biological Sci. and Plant Molecular Biology Center
Northern Illinois Univ., Dekalb, IL
An often powerful approach to characterizing the relationships among
plant taxa is to compare the nucleotide sequences of their ribosomal DNA.
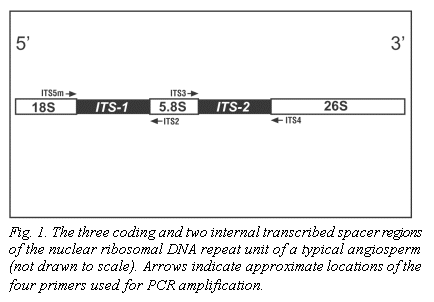
Nuclear ribosomal DNA (nrDNA) is organized as distinct chromosomal units that
are repeated thousands of times in most higher plant genomes. Each of these
units contains the genes that encode the 18S, 5.8S and 26S ribosomal RNA
subunits, as well as several different spacer DNA regions. The nucleotide
sequence variation found in both of the internal transcribed spacer regions
(ITS-1 and ITS-2, Fig. 1) is routinely used for the systematic analysis of
closely related taxa, at least in part due to the high rate of evolutionary
change characterizing these DNA regions (1).
 In our preliminary study of pea ITS regions (6), ITS-1 and ITS-2 DNA
sequence variation was assessed for five pairs of wild and cultivated pea taxa
selected to approximate the range of Pisum.
The objective of that investigation was to examine the similarity of the
sequences within paired accessions, the overall level of genetic variation found
across the entire genus, and the topological relationships established among the
five selected groups of taxa. It resulted in the following six observations: 1)
very close genetic affinities throughout Pisum, with P. fulvum
exhibiting the greatest degree of divergence, 2) support for the established
taxonomic categories of the genus based upon identical or near identical
sequences within group pairs, 3) the assignment of JI1794 as a “northern” humile,
4) the validity of northern and southern humile
as closely-related, but distinct, lines, 5) the apparent independent
evolution of a pea chromosomal translocation and 6) a close relationship between
elatius and the cultivated sativum.
Additionally, when Vicia montbrettii
was included as an outgroup to Pisum
in both the preliminary and present studies, phylogenetic analyses indicated
that P. fulvum remained not only the
most divergent pea taxon but also the most basal taxon relative to the sativum
group (data not shown).
In our preliminary study of pea ITS regions (6), ITS-1 and ITS-2 DNA
sequence variation was assessed for five pairs of wild and cultivated pea taxa
selected to approximate the range of Pisum.
The objective of that investigation was to examine the similarity of the
sequences within paired accessions, the overall level of genetic variation found
across the entire genus, and the topological relationships established among the
five selected groups of taxa. It resulted in the following six observations: 1)
very close genetic affinities throughout Pisum, with P. fulvum
exhibiting the greatest degree of divergence, 2) support for the established
taxonomic categories of the genus based upon identical or near identical
sequences within group pairs, 3) the assignment of JI1794 as a “northern” humile,
4) the validity of northern and southern humile
as closely-related, but distinct, lines, 5) the apparent independent
evolution of a pea chromosomal translocation and 6) a close relationship between
elatius and the cultivated sativum.
Additionally, when Vicia montbrettii
was included as an outgroup to Pisum
in both the preliminary and present studies, phylogenetic analyses indicated
that P. fulvum remained not only the
most divergent pea taxon but also the most basal taxon relative to the sativum
group (data not shown).
The goal of the present study is to extend the use of ITS variation as a
comparative tool to an additional 55 wild and cultivated pea taxa, both to
validate our preliminary findings among a more diverse sample of the genus and
to include previously unexamined pea types in these analyses.
Materials and Methods
Pisum
isolates 701-722 are from the Ben Ze’ev and Zohary (1973) collection (courtesy
of J.G. Waines), JI accessions are from the John Innes collection (courtesy of M.
Ambrose), cv. Alaska is from J. Mollema and Son, Inc. (Grand Rapids, MI) and cv.
(Morse’s) Progress #9 is from Ferry-Morse Seeds (Mountain View, CA). P.
sativum Syriacum was graciously provided by R. Jorgensen, and accessions
82-14n, A1078-234 and PI 179449 were kindly provided to this project by G. Marx
and N. Weeden.
DNA extraction, PCR amplification, gel purification, and ITS primers
(ITS2, ITS3, ITS4 and ITS5m) are described elsewhere (6).
DNA sequencing is performed with either an Applied Biosystems model 373
DNA sequencer or a Beckman Coulter CEQ 2000 XL DNA analysis system. Forward and
reverse DNA sequences are compared to resolve ambiguities using PC Gene software
and the resulting sequences are aligned with the Clustal X computer program.
Sequence data are analyzed using the PAUP computer package (7).
Results and Discussion
The pea 18S rRNA, ITS-1, 5.8S rRNA, ITS-2 and 26S rRNA regions examined
in this study contain 27, 238, 164, 213 and 22 alignable base pairs (bp),
respectively, totaling 664 bp (including 451 bp of spacer DNA) for all but one
of the 65 plants analyzed. The only exception to these results involves a P.
sativum Syriacum accession that contains an additional guanine at ITS-2
position number 582. Ambiguous or polymorphic pyrimidine and purine sites are
denoted by the IUPAC/IUB symbols “Y” and “R,” respectively. Of the 664
total bp sequenced for each of the individual plants, 640 (>96%) of these
sites are constant among the 64 pea taxa. Of the 451 ITS bp sequenced, 428
(>94%) of these sites are constant. Only 24 of the total sites are
polymorphic (and only 21 are parsimony informative), reaffirming both the very
close evolutionary relationships that must exist within the genus and the
limited ITS information available with which to differentiate pea taxa. In this
study, ITS-1 contains 14 of the polymorphic sites, as compared with nine found
for ITS-2 and one polymorphic site located just within the 5.8S rRNA coding
region (Table 1).
Table
1. Variable ITS sites for wild and cultivated taxa of pea.
|
|
Nucleotide
Position*
|
Number of Base Changes from
fulvum
|
GenBank
Acces-sions
numbers
|
|
ITS-1 ITS-2
|
|
111111111122222445566666
011233334903346570300023 358425895084607900001411
|
|
Pisum
fulvum
|
|
|
|
|
701
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AF305582
|
|
|
|
|
AF305920
|
|
702
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AF305583
|
|
|
|
|
AF305921
|
|
703
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143432
|
|
706
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143433
|
|
707
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143434
|
|
708
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143435
|
|
JI224
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143447
|
|
JI1006
|
GTTGGGCACCGACTGTTCTTGAAG
|
|
AY143451
|
|
Pisum
sativum
|
|
|
|
|
ssp.
humile (northern)
|
|
|
|
|
716
|
GTCGGGCGCTACCCACCCATGTAC
|
11
|
AF305586
|
|
|
|
|
AF305924
|
|
JI1794
|
GTCGGGCGCTACCCACCCATGTAC
|
11
|
AF305587
|
|
|
|
|
AF305925
|
|
ssp.
humile ( southern )
|
|
|
|
|
711
|
RYCRAACGCTACCCACCCATGAAC
|
12
|
AY143436
|
|
712
|
RYCRGACGCTACCCACCCATGAAC
|
11
|
AF305584
|
|
|
|
|
AF305922
|
|
713
|
RYCRAACGCTACCCAYCCATGAAC
|
12
|
AF305585
|
|
|
|
|
AF305923
|
|
714
|
RYCGGACGCTACCCACCCATGAAC
|
11
|
AY143437
|
|
ssp.
elatius
|
|
|
|
|
721
|
GCCGTACGYTACCCACCCATGTAC
|
14
|
AF305588
|
|
|
|
|
AF305926
|
|
722
|
GCCGTACGYTACCCACCCATGTAC
|
14
|
AF305589
|
|
|
|
|
AF305927
|
|
723
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143438
|
|
JI64
|
GCCGGACGCTACCCACCCATGTAC
|
13
|
AY143442
|
|
JI261
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143450
|
|
JI2201
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143455
|
|
ssp.
abyssinicum
|
|
|
|
|
JI2
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143441
|
|
JI130
|
GCCGGACGCTACCCACCCATGTAC
|
13
|
AY143444
|
|
JI225
|
GCCGGACGTTACCCACCCATGTAC
|
14
|
AY143448
|
|
JI2202
|
GCCGGACGTTACCCACCCATGTAC
|
14
|
AY143456
|
|
ssp.
sativum
|
|
|
|
|
JI196
Georgia
|
GCCGAAYGCTACCCACCCATGTAC
|
14
|
AY143463
|
|
JI228
Bolivia
|
RCCGAACGCTACCCACCCATGTAC
|
14
|
AY143466
|
|
JI245
Russia
|
GCCGAACGYTAYCCACCCATGTAC
|
14
|
AY143467
|
|
JI1035
Turkey
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143473
|
|
JI1057
AntioquiaI Chilena
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143474
|
|
JI1345
Mongolia
|
GCCGAACGYTACCCACCCATGTAC
|
14
|
AY143476
|
|
JI1428
(P. tibetanicum)
|
GCCGAACGYTACCCACCCATGTAC
|
14
|
AY143478
|
|
JI1835
Spain
|
GCCGAACGYTACCCACCCATGTAC
|
14
|
AY143481
|
|
JI2116
(P . speciosum)
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143482
|
|
JI2124
ponderosum
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
&Y143483
|
|
JI2265
Primitive Albanian
|
GCCGAAYGYTACCCACCCATGTAC
|
14
|
AY143484
|
|
JI2385(P.
sp. Yemen)
|
GCCGGACGCTACCCACCCATGTAC
|
14
|
AY143485
|
|
82-14n
|
GCCGAACGCTACCCACCCATGTAC
|
14
|
AY143457
|
|
JI185
Wiraig
|
GCCGAACGTTAYCCACCCATRTAC
|
15
|
AY143462
|
|
JI263
Balkans
|
ACCGAACGYTAYCCACCCATGTAC
|
15
|
AY143469
|
|
JI264
Greece
|
RCCGAACGTTAYCCACCCATGTAC
|
15
|
AY143470
|
|
JI711
Austrian Winter
|
ACCGAACGCTACCCACCCATGTAC
|
15
|
AF305590
|
|
|
|
|
AF305929
|
|
JI787
Minerva
|
GCCGAATGYTACCCACCCATGTAC
|
15
|
AY143471
|
|
JI1372
Mummy Pea
|
ACCGAACGYTACCCACCCATGTAC
|
15
|
AY143477
|
|
JI1758
Nepal
|
GCCGAACGTTAYCCACCCATRTAC
|
15
|
AY143480
|
|
712438
Partridge
|
ACCGAACGYTAYCCACCCATGTAC
|
15
|
AY143486
|
|
Alaska
|
ACCGAACGYTACCCACCCATGTAC
|
15
|
AF305202
|
|
|
|
|
AF305928
|
|
PI179449
|
RCCGAACGTTACCCACCCATGTAC
|
15
|
AY143440
|
|
Syriacum
|
GCCGAAYGTTACCCACCCATGTAC
|
15
|
AY143459
|
|
JI85
Afghanistan
|
ACCGAACGTTACCCACCCATGTAC
|
16
|
AY143443
|
|
JI156
Sudan
|
ACCGAACGTTACCCACCCATGTAC
|
16
|
AY143445
|
|
JI159
Ethiopia
|
ACCGAACGTTAYCCACCCATRTAC
|
16
|
AY143460
|
|
JI181
Keerau Pea
|
GCCGAACGTTATCCACCCATRTAC
|
16
|
AY143461
|
|
JI207
choresmicum
|
ACCGAACGTTAYCCACCCATRTAC
|
16
|
AY143464
|
|
JI209
arvense
|
ACCGAACGTTAYCCACCCATGTAC
|
16
|
AY143465
|
|
JI250
(P. jomardii)
|
ACCGAACGTTAYCCACCCATGTAC
|
16
|
AY143468
|
|
JI1578
China
|
ACCGAACGTTAYCCACCCATGTAC
|
16
|
AY143479
|
|
Progress
#9
|
ACCGAACGTTAYCCACCCATGTAC
|
16
|
AY143458
|
|
M078-234
|
ACCGAACGTTACCCACCCATGTAC
|
16
|
AY143439
|
|
JI1033
India
|
GCCGAACGTTATCCACCCATATAC
|
17
|
AY143472
|
|
JI1089
Syriacum
|
ACCGAACGTTATCCACCCATRTAC
|
17
|
AY143475
|
|
Inconsistent
assignments :
|
|
|
|
|
JI241
(1)
|
ACCGGACGTTACCCACCCATGTAC
|
15
|
AY143449
|
|
JI198
(2)
|
GCCGAACGTTACCCACCCATGTAC
|
15
|
AY143446
|
|
JI1398
(2)
|
ACCGAACGTTACCCACCCATGTAC
|
16
|
AY143453
|
|
JI1096
(3)
|
ATCGAACGCTACTCACCTACGTTC
|
18
|
AY143452
|
|
JI2055
(3)
|
GTCGAACGCTACTCACCTACGTTC
|
17
|
AY143454
|
* In the 5'->3' direction (see Fig. 1)
beginning with those bases nearest primer ITS5m. Position 267 is assigned to the
5.8S rRNA coding region.
(1)
JI241 is listed as ssp. humile, but it displays ssp. sativum ITS
characteristics.
(2)
JI198 and JI1398 are listed as ssp. elatius, but they display ssp.
sativum ITS characteristics.
(3)
JI1096 and JI2055 are listed as ssp. elatius, but they display unique ITS
variation at several sites
Parentheses
around four JI accessions indicate taxonomic nomenclature not supported in this
table.
A compilation of the 24 variable nrDNA sites is delineated for all 65 pea
taxa in Table 1, accompanied by corresponding GenBank accession numbers for the
retrieval of complete sequences. The table is organized in accordance with the
two commonly recognized species of pea (2-4), the more divergent P. fulvum and the typically cultivated P. sativum. The former is represented by eight identical nrDNA
sequences, while the latter is differentiated as four subspecies: humile,
elatius, abyssinicum and sativum.
Subspecies humile is further subdivided by northern and southern populations as
described by (2). There are five pea accessions characterized as questionable
taxonomic assignments solely based on their nrDNA variation, and there are also
differences distinguishing from one another the two “Syriacum” accessions
surveyed. The four subspecies and 52 assigned accessions of P.
sativum are further arranged in Table 1 by the number of unambiguous base
changes each possesses relative to the invariant P.
fulvum accessions. The number of base differences separating fulvum
from the 52 sativum accessions ranges
from 11 to 17, with 10 of these sites being unique to fulvum. JI1096, an elatius
accession displaying unique ITS variation at several sites, shows 18 base
differences with fulvum. The
subdivisions of P. sativum are listed
in the following order based on their base pair differences with fulvum:
northern humile (11 base changes), southern humile (11-12 base changes), elatius
and abyssinicum (13-14 base changes
each), and sativum (14-17 base changes).
Named cultivars of sativum usually
display 15 or 16 base changes.
A Neighbor Joining (NJ) distance analysis of these data is presented in
Fig. 2 to provide a basic illustration of the associations suggested in Table 1,
while also including such influences as the multiple polymorphisms found at
ITS-1 sites 132 and 234. No attempt is made, however, to infer evolutionary
relationships among the 65 taxa, given the relatively few parsimony informative
sites available to the analysis. In the figure,
only fulvum, northern and southern humile
and a pair of elatius accessions
maintain distinct group associations. Ten of the 21 parsimony informative sites
differentiate fulvum from the much
larger sativum ingroup. Within sativum,
the two northern humile accessions
display completely identical nucleotide sequences (at 664 sites), while the
southern humile differ at a single
site and show ambiguity at several others. Only two elatius
accessions (JI 1096 and JI 2055), displaying four unique sites and the largest
overall numbers of sequence differences with fulvum, group separately from the remaining sativum subspecies. These remaining accessions group roughly based
on possessing 14, 15 or 16 base differences with fulvum. Most of the other elatius
and all four abyssinicum are found in
the first group, along with approximately a dozen sativum and the single questionable humile accession. The latter two groups principally comprise sativum,
including most of the named cultivars.
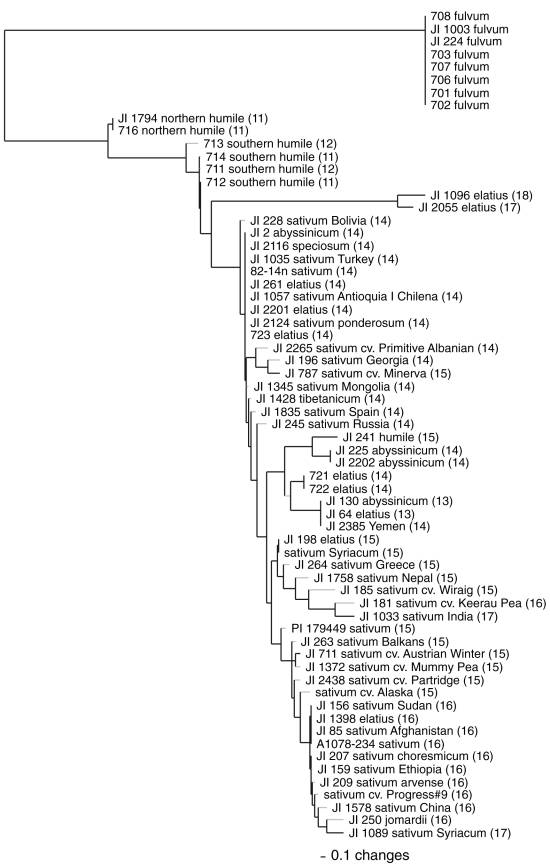
Fig.
2. Neighbor Joining phylogram
of 65 wild and
cultivated pea taxa based on
24 variable nrDNA sites (23
ITS
and one 5.8S rRNA).
Number of base pair
differences
from P.
fulvum
(as shown in Table 1) are indicated in parentheses. Branch length distances are
drawn with reference to the 0.1 length standard.
According to Fig. 2, elatius and abyssinicum
are the closest taxa to the cultivated sativa,
despite the fact that northern humile
has been postulated the closest wild progenitor of the cultivated pea based in
part on a shared chromosomal translocation (2) and detailed chloroplast studies
(5). Other, larger data sets (not shown) place northern humile closer to sativum,
but they do not support northern humile
as the taxon closest to the cultivars. Thus, the present study largely supports
the conclusions from our previous work (6): generally very close relationships
within Pisum, with P.
fulvum clearly displaying the greatest divergence; JI 1794 classified as a
“northern” humile; northern and southern humile
as closely-related, but distinct, taxa; and the independent evolution of a pea
chromosomal translocation. The study also supports distinct taxonomic categories
for fulvum and for northern and
southern humile; however, the ITS
sequence variation obtained from this investigation is too limited to separate
unambiguously the very close relationships among elatius,
abyssinicum and sativum. Further
efforts are needed to resolve these relationships and to clarify the taxonomic
assignments of the few questionable accessions addressed in this study.
Acknowledgement:
We thank Scott Grayburn for his DNA sequencing skills. This work was supported
by funds from the Department of Biological Sciences and the Plant Molecular
Biology Center, Northern Illinois University.
1.
Baldwin, B.G., Sanderson, M.J., Porter, J.M., Wojciechowski, M.F.,
Campbell, C.S. and Donoghue, M.J. 1995.
Ann. Mo. Bot. Gard. 82: 247-277.
2.
Ben Ze’ev, N. and Zohary, D. 1973.
Israel J. Bot. 22: 73-91.
3.
Hoey, B.K., Crowe, K.R., Jones, V.M. and Polans, N.O.
1996. Theor. Appl. Genet. 92: 92-100.
4.
Marx, G.A. 1977.
In: Sutcliffe, J.F. and
Pate, J.S. (eds.). Physiology of
the Garden Pea. Academic Press, New
York, pp 21-43.
5.
Palmer, J.D., Jorgensen, R.A. and Thompson, W.F.
1985. Genetics 109: 195-213.
6.
Saar, D.E. and Polans, N.O. 2000.
Pisum Genetics 32: 42-45.
7.
Swofford, D.L. 1998.
PAUP, Version 4.0b4a. Sinauer Associates, Sunderland, MA.
 In our preliminary study of pea ITS regions (6), ITS-1 and ITS-2 DNA
sequence variation was assessed for five pairs of wild and cultivated pea taxa
selected to approximate the range of Pisum.
The objective of that investigation was to examine the similarity of the
sequences within paired accessions, the overall level of genetic variation found
across the entire genus, and the topological relationships established among the
five selected groups of taxa. It resulted in the following six observations: 1)
very close genetic affinities throughout Pisum, with P. fulvum
exhibiting the greatest degree of divergence, 2) support for the established
taxonomic categories of the genus based upon identical or near identical
sequences within group pairs, 3) the assignment of JI1794 as a “northern” humile,
4) the validity of northern and southern humile
as closely-related, but distinct, lines, 5) the apparent independent
evolution of a pea chromosomal translocation and 6) a close relationship between
elatius and the cultivated sativum.
Additionally, when Vicia montbrettii
was included as an outgroup to Pisum
in both the preliminary and present studies, phylogenetic analyses indicated
that P. fulvum remained not only the
most divergent pea taxon but also the most basal taxon relative to the sativum
group (data not shown).
In our preliminary study of pea ITS regions (6), ITS-1 and ITS-2 DNA
sequence variation was assessed for five pairs of wild and cultivated pea taxa
selected to approximate the range of Pisum.
The objective of that investigation was to examine the similarity of the
sequences within paired accessions, the overall level of genetic variation found
across the entire genus, and the topological relationships established among the
five selected groups of taxa. It resulted in the following six observations: 1)
very close genetic affinities throughout Pisum, with P. fulvum
exhibiting the greatest degree of divergence, 2) support for the established
taxonomic categories of the genus based upon identical or near identical
sequences within group pairs, 3) the assignment of JI1794 as a “northern” humile,
4) the validity of northern and southern humile
as closely-related, but distinct, lines, 5) the apparent independent
evolution of a pea chromosomal translocation and 6) a close relationship between
elatius and the cultivated sativum.
Additionally, when Vicia montbrettii
was included as an outgroup to Pisum
in both the preliminary and present studies, phylogenetic analyses indicated
that P. fulvum remained not only the
most divergent pea taxon but also the most basal taxon relative to the sativum
group (data not shown).
