|
Pisum Genetics |
Volume 26 |
1994 |
Research Reports |
pages 21-23 |
Late flowering in mutant E54LF results from mutation of sn to Sn
|
Murfet, I.C.
LaRue, T.A.
|
Department of Plant Science, University of Tasmania Hobart , Tasmania 7001, Australia Boyce Thompson Institute Ithaca , NY 14853-1801 , USA |
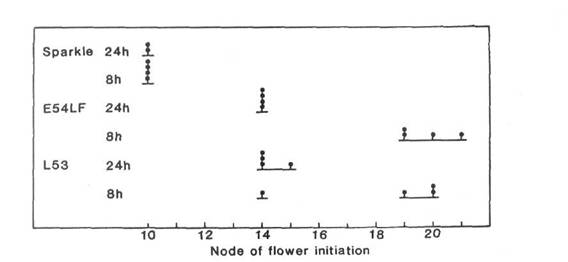
Cultivar Sparkle is an early maturing freezer pea (Roger Bros. Seed Co., Twin Falls ID, USA). While using induced mutagenesis to identify symbiosis genes (4), we isolated E54 with only a few nodules in the M2 screen of seedlings treated as M1 seeds with 1% ethyl methane sulphonic acid for 30 min. E54 was noted to flower later than the parental line Sparkle. After E54 and Sparkle were crossed, low nodulation and late flowering segregated independently in the F2. We report here on the identity and characterisation of E54LF, a true breeding line with late flowering and normal nodulation selected from this F2. Previous work (11) had shown that Sparkle belongs to the early day-neutral flowering class [class ED of Murfet (6)] and has flowering genotype lf e sn Dne Ppd hr [the flowering genes in pea are reviewed in (9)]. Our tests (Fig. 1) show E54LF belongs to the late flowering class with a limited quantitative response to photoperiod [class L of Murfet (6) or K of Marx (5)]. Counting from the first scale leaf as node 1, the flowering node of E54LF increased from 14 under continuous light to 20 under an 8 h short day photoperiod. In contrast, the initial line Sparkle flowered about node 10 regardless of the photoperiod.
The ability to respond to photoperiod is conferred in pea by the joint presence of the three complementary dominant genes Sn (2, 7), Dne (3) and Ppd (1). Thus, given that Sparkle has genotype lf e sn Dne Ppd hr, it may be inferred that E54LF has resulted from a back mutation of sn to Sn. This conclusion is supported by the results of our tests. First, line E54LF has flowering characteristics closely comparable with those of reference line 53 (6, 7) known to have genotype lf e Sn Ppd Dne hr (Figs 1 and 2). Second, the F1 of cross E54LF x L53 has a very similar phenotype to the two parents (Fig. 2). Third, the F1 and F2 data of cross E54LF x Sparkle (Fig. 2) indicate a monohybrid difference with dominance of the allele for late flowering. The F2 segregation was clear with 40 late and 8 early segregants in good accordance with a 3:1 ratio (P > 0.1).
As a further test we crossed E54LF, which has white flowers (a), with the red flowered (A) Hobart line, L73 (Lf E sn Dne Ppd hr). The flowering locus Lf shows fairly close linkage with marker A in linkage group I (distance about 10 cM; 7). Under an 8 h photoperiod, the F1 was late flowering and the F2 segregated clearly for the Sn - sn pair of alleles with 16 sn segregants flowering 34-38 days after sowing and 48 Sn segregants flowering 51-76 days after sowing (perfect 1:3 ratio). Among the F2 day neutral class, red flowered (A) plants had a mean flowering node of 11.67 ± 0.14 (n=12) compared with 10.75 ± 0.25 (n=4) for the white flowered (a) plants (difference significant, P < 0.01). Among late flowering segregants, A plants had a mean flowering node of 25.27 ± 0.67 (n=37) and a plants a mean of 22.09 ± 1.50 (n=ll) (difference significant, P < 0.05). These results are in accordance with the view that E54LF carries the lf allele in coupling phase with a and that the late flowering in E54LF has not resulted from a back mutation at the Lf locus.

Fig. 1. Node of flower initiation for the initial line cv. Sparkle (flowering genotype lf e sn Dne Ppd hr; 10), mutant line E54LF and reference line L53 (lf e Sn Dne Ppd hr; 5, 6) under an 8 h short day photoperiod (8 h daylight, 16 h dark) or a 24 h long day photoperiod (8 h daylight, 16 h incandescent light at 3 mmol m-2s-1). The L53 plant flowering at node 14 in short days represents one of the impenetrant types which occur with low frequency in this line (see 6).
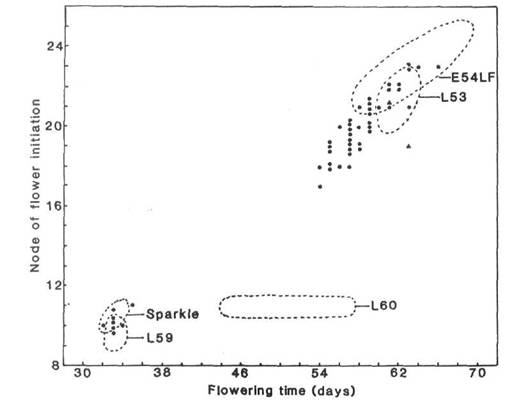
Fig. 2. Node of flower initiation plotted against days to first open flower for the F1 (▲) and F2 (●) of cross E54LF x Sparkle, parental lines E54LF and Sparkle, and Hobart control lines (see 6) of known flowering genotype: L59 lf E sn Dne Ppd hr, standard early day neutral line; L60 lf E Sn Dne Ppd hr, standard early photoperiodic line; L53 lf e Sn Dne Ppd hr, reference L-class line (late with quantitative response to photoperiod). The data for the parental and control lines are encircled by the broken lines; n=4. The data for the standard L-class line L24 (Lf e Sn Dne Ppd hr) and the F1 of cross E54LF x L53 are not plotted but the co-ordinates are as follows: L24 nodes 28-35 and days 73-81; E54LF x L53 F1 nodes 21-23 and days 60-64. Photoperiod 8 h.
We conclude that E54LF has resulted from mutation of sn to Sn. The Sn gene has proved fairly resistant to mutation and only three flowering mutants, including E54LF, have so far been traced to this locus (1, 8). In contrast, the Lf gene is substantially more susceptible to mutation and 21 flowering mutants have now been identified at this locus (see 10). The two genes also show another difference in behaviour. All 21 mutations at the Lf locus have been in the forward direction leading to earlier flowering. In contrast, 2 of the 3 mutations at the Sn locus have been back mutations to the dominant allele and have resulted in restoration of the wild type photopenodic habit. In one instance the back mutation arose during callus culture (8) while in the present case the mutation was induced following treatment with ethyl methane sulphonic acid.
Arumingtyas, E.L. and Murfet, I.C. 1994. J. Hered. 85:12-17.
Barber, H.N. 1959. Hered. 13:33-60.
King, W.M. and Murfet, I.C. 1985. Ann. Bot. 56:835-846.
Kneen, B.E. and LaRue, T.A. 1988. Plant Sci. 58:177-182.
Marx,G.A. 1986. BioSci. 18:505-506.
Murfet, I.C. 1971. Hered. 26:243-257.
Murfet, I.C. 1971. Hered. 27:93-110.
Murfet, I.C. and Ezhova, T.A. 1991. Pisum Genet 23:19-25.
Murfet, I.C. and Reid, J.B. 1993. In Peas -
Genetics, Molecular Biology and
Biotechnology, Eds R. Casey and D.R. Davies, CAB International, Wallingford,
U.K.
pp. 165-216.
Taylor, S.A. and Murfet, I.C. 1993. Pisum Genet 25:60-63.
Weeden, N.F., Kneen, B.E. and Murfet, I.C. 1988. Pisum Newsl. 20:49-51.