|
Pisum Genetics |
Volume 24 |
1992 |
Research Reports |
pages 83-85 |
Internode length in Pisum. Interaction of genes lhi, la and crys.
|
Swain, S.M. and Reid, J.B. |
Department of Plant Science, University of Tasmania Hobart, Tasmania 7001, Australia |
In peas, a range of internode length mutations that affect either gibberellin (GA) synthesis or the response to GA have been identified. GA synthesis mutants have been identified at the Le, Na, Ls and Lh loci, and are characterised by a dwarf stature (reduced internode lengths) resulting from a reduction in the level of the biologically active GA1 in the shoot compared with wild-type plants (5, 9, 10). At the Lh locus, two mutant alleles have now been identified: lh (7) and lhi (11). The lhi allele also reduces GA levels in the developing seeds, compared with its wild-type progenitor, Torsdag (Lh) (S.Swain, J.B. Reid and J.J. Ross, unpublished data). The reduced levels of GA1 and GA3 in young ovules is believed to account for the increased seed abortion and reduced seed yield of homozygous lhilhi plants (11).
Pea genotypes with altered response to GA include the la crys gene combination. Plants homozygous for both the la and crys alleles exhibit the slender phenotype (long, thin internodes, 1, 2, 8) regardless of endogenous GA levels (3, 6). Hence, the La and Cry gene-products are thought to act at or after GA perception in the transduction chain linking GA1 levels to changes in internode length (6).
A GA response mutant that is epistatic to the lhi allele in developing seeds would lend support to the hypothesis that GA's play an important role in seed development Therefore, in an attempt to provide further evidence for a physiological role of the GAs in shoots and developing seeds, the interaction between the la crys gene combination and the lhi allele was investigated.
Materials and Methods
The pure lines of Pisum sativum L. used during this work are held in the collection at Hobart, Australia. The dwarf line NGB5843 (lhi La Cry) was derived from the wild-type tall cv. Torsdag (Lh La Cry) by Dr K. K. Sidorova (Novosibirsk, Russia). The slender line 197 (Lh la crys) was derived from a cross between Hobart line 133 and NGB1766. Lines NGB5843 and 197 are both homozygous dominant at the internode length loci Le, Ls, Na, Lka, Lkb, Lkc, Lkd, Lv, Lk, Lm and Sln. Further details about the phenotypes and genotypes of these lines can be found in papers 7 and 11.
Plants were grown in a 1:1 (v:v) mixture of vermiculite and 10 mm dolerite chips topped with 2-3 cm of potting mix in 14 cm slim-line pots (2 per pot) or plastic tote boxes (41 x 32 cm). All plants were grown in an 18 h photoperiod and provided with nutrient solution (Aquasol) once a week. Counting of nodes started from the first scale leaf as node 1.
All F1, F2, F3, F4 and F5 plants resulting from the cross between lines 197 (Lh la crys) and NGB5843 (lhi La Cry) were initially grown in a heated glasshouse (9). Some of the F3 plants were transferred 3 weeks after planting to a controlled environment cabinet with a day/night temperature regime of 25/20°C where light was provided by a mixed fluorescent (Thorn 40W cool white tubes) and incandescent (Mazda 100W pearl globes) source (200 mmol m-2 s-1 at pot top).
Results and Discussion
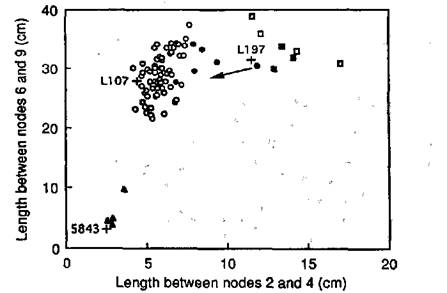
The F1 of the cross between lines NGB5843 (dwarf; lhi La Cry) and 197 (slender; Lh la crys) had a tall phenotype and the F2 (Fig. 1) segregated into three classes: tall, dwarf and slender. Seeds from some of the tall F2 plants (solid circles, Fig. 1) and some of the slender F2 plants (solid squares, Fig. 1) were grown-on to confirm their genotypes. One phenotypically slender F2 plant was found to be genetically tall since its progeny segregated to give 5 tall and 1 slender F3 plant (data not shown). Three phenotypically slender F2 plants bred true in F3. The remaining four slender F2 plants could not be progeny tested because they produced only parthenocarpic pods and no seeds. This is a pleiotropic effect of the slender gene combination (6) and is consistent with these plants having genotype la crys. All seeds from the four dwarf F2 plants were grown on in the F3. One dwarf F2 plant produced in F3 13 dwarf and 1 slender offspring. Hence, the genotype of this slender F3 plant was lhi la crys, demonstrating that the slender phenotype is expressed on a lhi background. This result is similar to that obtained for the na la crys and le la crys genotypes (3, 4, 6) and demonstrates that the la crys gene combination is epistatic to the na, le and lhi genes in young shoots, and is expressed regardless of GA levels in this tissue. Plants of genotype lhi la crys also developed parthenocarpic pods in subsequent generations.
Having established that plants homozygous for lhi, la and crys have a slender phenotype, the expected F2 ratio is 45 tall: 15 dwarf: 4 slender. The observed F2 numbers of 74 tall, 4 dwarf and 7 slender plants (Fig. 1) are not in agreement with the expected ratio (c2 = 16.64, P<0.001). However, the number of slender plants agreed with expected results when compared with the total number of F2 plants (c2 for 1:15 = 0.57, P>0.3). In contrast, there was a significant deficiency in the observed number of dwarf compared with tall F2 plants (c2 for 1:3 = 16.42, P<0.001) as observed in other crosses segregating for the Lh and lhi alleles (11).

Fig. 1. Internode lengths for the F2 resulting from a cross between line 197 (Lh la crys) and NGB5843 (lhi La Cry), showing segregation into slender (□, ■), tall (○, ●) and dwarf (▲) phenotypic classes. Solid symbols represent plants bred-on to confirm their genotype. Means for parental lines 197 and NGB5843, and for the wild-type cv. Torsdag (Lh La Cry), are indicated by crosses (n≥5). Photoperiod 18 h.
Eleven of the dwarf F3 plants, from the family containing 1 slender plant, were allowed to self-pollinate in an 18 h photoperiod with a day/night temperature regime of 25/20°C. No significant deviation from expected results was observed in any of the resulting progeny. Four F4 families segregated in accordance with a ratio of 3 dwarf to 1 slender plant (totals: 83 dwarf, 26 slender, c2 for 3:1 = 0.08, P>0.7). Three F4 families segregated in accordance with a ratio of 15 dwarf to 1 slender plant (totals: 73 dwarf, 4 slender, c2 for 15:1 = 0.15, P>0.7). One F4 family segregated in accordance with both a 3:1 and a 15:1 ratio (16 dwarf, 2 slender). Three F4 families bred true.
Seeds possessing genotype lhilhi have previously been shown to weigh less than Lh- seeds (11). Since the la crys gene combination is epistatic over the lhi allele in shoots, the possible epistasis over final seed weight of lhi seeds was examined. Six dwarf plants from F4 families containing approximately 1/4 slender segregates were allowed to self-pollinate in a glasshouse during winter (day and night temperatures of between 20-25 and 10-15°C, respectively). The resulting seeds were weighed immediately prior to sowing to determine if the slender F5 plants (segregating in four families) developed from heavier seeds than the dwarf F5 plants. However, no significant difference (t=0.41, P>0.5) was found between the weight of seeds which gave rise to slender plants (0.298±0.010 g/seed, n=13) and those which gave rise to dwarf plants (0.293±0.007 g/seed, n=48). Hence, no evidence was found for epistasis of the la crys gene combination over the lhi allele in developing seeds.
In conclusion, the la crys gene combination is epistatic over the lhi allele in young shoots. The results support the hypothesis that the slender phenotype (long, thin basal internodes) is independent of endogenous GA levels since the lhi allele is thought to block GA production before GA12-aldehyde, the precursor to all GAs in peas (11).
Acknowledgements. We thank Katherine McPherson for technical assistance and the Australian Research Council for financial support.
deHaan, H. 1927. Genetica 9: 481-497.
deHaan, H. 1930. Genetica 12: 321-439.
Ingram, T.J. and J.B. Reid. 1987. J. Plant Growth Regul. 5: 235-243.
Lamm, R. 1937. Hereditas 23: 38-48.
Potts, W.C. and J.B. Reid. 1983. Physiol. Plant. 57: 448-454.
Potts, W.C, J.B. Reid and I.C. Murfet. 1985. Physiol. Plant. 63: 357-364.
Reid, J.B. 1986. Ann. Bot. 57: 577-592.
Reid, J.B., I.C. Murfet and W.C. Potts. 1983. J. Exp. Bot. 34: 349-364.
Reid, J.B. and W.C. Potts. 1986. Physiol. Plant. 66: 417-426.
Ross, J.J., J.B. Reid, P. Gaskin and J. MacMillan. 1989. Physiol. Plant. 76: 173-176.
Swain, S.M. and J.B. Reid. 1992. Physiol. Plant. (in press).