|
Pisum Genetics |
Volume 24 |
1992 |
Research Reports |
pages 75-78 |
Expression of det (determinate) in genotypes Lfd, Lf, lf and lfa
|
Murfet, I.C.
|
Department of Plant Science University of Tasmania Hobart, Tas. 7001, Australia |
Plants homozygous for the mutant allele det (determinate) terminate growth after producing only a small number of reproductive nodes (1, 2, 9, 11). Singer et al. (10) have shown that det plants are not botanically determinate because the apical meristem simply ceases growth and the final inflorescence is still formed by an axillary meristem. The interval between the onset of flower initiation and termination of apical activity in det plants is markedly influenced by the genotype at the Lf (late flowering) locus (8). Plants with genotype Lf/- usually produced only one or two normal reproductive nodes, i.e. nodes with a normal pinnate leaf subtending an axillary inflorescence, whereas lfa/lfa plants produced 3-6 normal reproductive nodes before terminating. In that study, all det/det plants produced at least nine leaves (2 scale leaves + 7 foliage leaves). Thus there is scope for lfa det plants to produce several reproductive nodes since allele lfa confers the potential for very early flowering (5) and such plants sometimes flower as early as node 5.
Four alleles, lfa, lf, Lf and Lfd are known at the Lf locus (3, 5) and they determine minimum flowering nodes of 5, 8, 11 and 15, respectively (6, 7). The present study was designed to see whether or not there is a progressive decline in the number of reproductive nodes produced by det plants as the sequence lfa, lf , Lf , Lfd is ascended.
Materials and Methods
Two Hobart lines with genotype lf or Lfd and a day neutral (sn), indeterminate (Det) habit, were crossed with day neutral, det segregates with genotype or lfa Lf chosen from the F4 of a cross between Hobart line L69 (lfa sn Det) and John Innes line JI1358 (Lf Sn det, see 8). Three crosses were made: cross 771 between line L68 (lf sn Det) and an F4 plant with genotype lfa sn det, cross 772 between line L89 (Lfd sn Det) and a plant with genotype lfa sn det, and cross 773 between line L89 (Lfd sn Det) and a plant with genotypeLf sn det. Lines 68 and 89 have coloured flowers (A) and the det parents had white flowers (a). The A and Lf loci are linked (distance about 10 cM; e.g. 3, 5) and the coupling phase arrangement was included to assist with resolution of segregation for the Lf alleles. The F2 generation from the three crosses did not contain sufficient det/det segregates to permit firm conclusions to be drawn. Hence F3 progenies from all det/det F2 segregates were used to provide the data in Table 1 and Fig. 1.
The plants were grown, one per pot, in 14 cm slim line pots in a 1:1 (v:v) mixture of dolerite chips (10 mm) and vermiculite topped with sterilized peat-sand potting mixture. Nutrient (Total Growth Nutrient, R&D Aquaponics, Sydney) was applied once a week. The plants received 8 h of daylight per day at a temperature generally around 18-22°C. They were then moved to 'night' compartments held at 16°C where the photoperiod was extended to 18 h by 10 h of light from a mixed fluorescent (40 W cool white)/incandescent (40 W tungsten bulbs) source providing 55 mmols m-2 s-1 at pot top.
The data were collected wholly from main shoots. The number of reproductive nodes was taken as the number of nodes to bear a normal pinnate leaf subtending an inflorescence. The cross segregating for the Lfd-lfa pair of alleles (cross 772) gave a discrete two-class segregation for flowering node. The flowering node distributions in the crosses for the lf-lfa (cross 771) and Lfd-Lf (cross 773) pairs were continuous but with obvious minimum frequency regions, and arbitrary cuts were made between the nodes shown in Table 1.
Results and Discussion
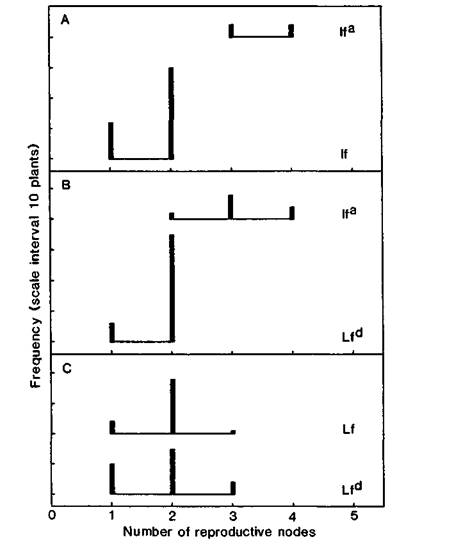
The results (Table 1 and Fig. 1) show that lfa det plants had more reproductive nodes than any of the other three Lf genotypes. Comparison between data for two different crosses is not strictly valid because the genetic background differs among the crosses. Nevertheless, there was no significant difference in number of reproductive nodes among the three genotypes lf, Lf and Lfd, or any indication of progressive change from lf, through Lf to Lfd. These three genotypes mostly produced no more than two reproductive nodes before terminating, although cross 773 (Fig. 1C) produced a few plants with three reproductive nodes. In contrast, lfa plants generally produced at least three reproductive nodes (Fig. 1A and B). Three out of 22 lfa plants produced 9 leaves before terminating while the remaining 19 plants (86%) produced 10 or 11 leaves. These results confirm the previous observation (8) that det plants always produce at least 9 leaves. In contrast to data obtained previously from cross L69 x JI1358 (8), there were no examples of lfa det plants with 5 or 6 reproductive nodes, but neither were there any plants flowering as early as nodes 5 or 6.
The lfa allele breaks the usual relationship between the onset of flower initiation and the termination of meristem activity in det plants. However, it is not clear whether the extended interval between initiation and termination results from an interaction with lfa itself or whether it is a consequence of very early flower initiation in the lfa material tested (background sn Dne) and a fixed minimum node limit for expression of det. This question should be resolved by examining det expression in genotype lfa e Sn Dne hr where flower initiation may commence well above the apparent 9-node-limit for expression of det (7). An aberrant F3 plant from cross 771 was excluded from the analysis in Table 1 and Fig. 1, but it may provide information relevant to the above issue. This plant had an abortive flower bud at node 10, a vegetative bud in the leaf axil at node 11, and normal leaves with flowers in their axils at nodes 12, 13 and 14; flower colour was white (a). This aberrant plant probably represents one of the rare lfa "escapes" from the 5-8 node region reported previously (6). If so, lfa det plants flowering above node 9 may also express an extended interval between the onset of flower initiation and meristem termination. This aberrant plant also raises a further issue. Is expression of the det program irrevocably triggered by the laying down of the first flower initial or does reversion to the production of vegetative axillary buds reset the clock? If the former situation holds, are intervening vegetative nodes counted by the det program? The occurrence of vegetative reversion in peas is well known in certain circumstances, e.g. in early photoperiodic (EI) and impenetrant late types (3), and in late genotypes induced to flower early by grafting to promotive stocks (4). Examination of the expression of det in such plants could help resolve these questions.

Fig. 1. Distribution of number of reproductive nodes (A) for lfa/lfa and lf/- segregates in the F3 of cross 771, (B) for lfa/lfa and Lfd/- segregates in the F3 of cross 772, and (C) for Lf/Lf and Lfd/- segregates in the F3 of cross 773. All plants det/det sn/sn. Photoperiod 18 h.
Table 1. The effect of segregation for lf-lfa (cross 771), Lfd-lfa (cross 772) and Lfd-Lf (cross 773) on the number of reproductive nodes produced by det/det plants.
|
Cross |
Genotype |
No of reproductive nodes |
Node of flower initiation (range) |
||
|
Mean |
SE |
n |
|||
|
771 F3 |
lfa det |
3.50 |
0.19 |
8 |
7-8 |
|
|
lf det |
1.71 |
0.07 |
42 |
9-13 |
|
772 F3 |
lfa det |
3.14 |
0.18 |
14 |
7-8 |
|
|
Lfd det |
1.85 |
0.06 |
41 |
16-23 |
|
773 F3 |
Lf det |
1.87 |
0.10 |
23 |
13-17 |
|
|
Lfd det |
1.79 |
0.13 |
29 |
18-23 |
Acknowledgements. I thank Dr Susan Singer for comments on the manuscript and the Australian Research Council for financial support.
Makasheva, R.Kh. and Drozd, A.M. 1987. Pisum Newsl. 19:31.
Marx, G.A. 1986. Pisum Newsl. 18:45-48.
Murfet, I.C. 1971. Heredity 27:93-110.
Murfet, I.C. 1971. Aust. J. Biol. Sci. 24:1089-1101.
Murfet, I.C. 1975. Heredity 35:85-98.
Murfet, I.C. 1978. Pisum Newsl. 10:48-52.
Murfet, I.C. 1985. In Handbook of Flowering Vol. 4. Ed A.H. Halevy, CRC Press, Boca Raton, pp. 97-126.
Murfet, I.C. 1989. Pisum Newsl. 21:44-47.
Popova, I.A. 1975. Trudy po selektsii i semenovodstvu ovoshchnykh kultur VNIISSOC 3:66-72.
Singer, S.R., Hsiung, L.P. and Huber, S.C. 1991. Am. J. Bot. 77:1330-1335.
Swiecicki, W.K. 1987. Pisum Newsl. 19:72-73.