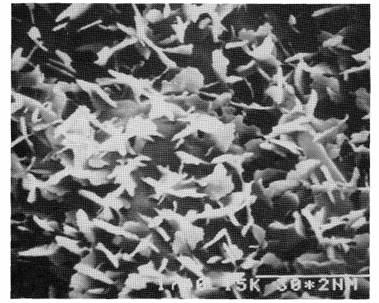
Fig. 1. Line R9: adaxial side of the leaf; x 10000; the bar above 30* 2 nm = 3 mm.
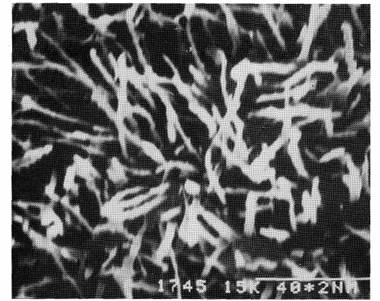
Fig. 2. Line R9: abaxial side of the leaf; x7500.
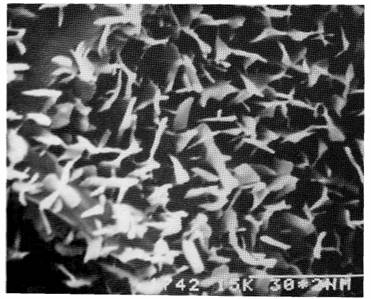
Fig 3. Line waxy2: adaxial side of the leaf; x 10000.
|
Pisum Genetics |
Volume 24 |
1992 |
Research Reports |
pages 60-63 |
Two waxless mutants of somaclonal origin in pea
|
Kovalenko, O.V. and Ezhova, T. A. |
Department of Genetics Moscow State University Moscow 119899, Russia |
Two monogenic recessive waxless mutants were obtained from long-term callus cultures of pea cv. Ranny Zeleny (1). They were named as waxy1 and waxy2. This paper reports on the distribution and ultrastructure of their wax cover and the results of linkage tests for the waxy1 and waxy2 mutations.
Materials and Methods
The ultra-structure of epicuticular wax on both sides of untreated leaflets was studied by scanning electron microscopy using a Hitachi S-405A microscope. Mutant lines waxy1 and waxy2 and the initial cultivar, Ranny Zeleny, carried the genes i, a, Af, lf, Le and R. To make crosses, the following marker lines were used: R1 (Lf; R1 is a regenerant line obtained from a callus culture of Ranny Zeleny), "moustached" line (I, A, af, le), L1072 (wb) and Hobart line L63 (I, A, le, r). The joint segregation c2 was obtained using a 2 x 2 contingency table and the recombination fraction was calculated using the product ratio method.
Results
Surface examinations were made on plants grown in the quite warm and dry 1991 summer field conditions. These conditions promoted fully the production of wax. It was found that waxy1 plants had a normal wax cover on the upper surface of the leaflets but lacked wax on the under surface of the leaflets and both sides of the stipules; the stem carried a reduced quantity of wax. The waxy2 plants were covered with a normal quantity of wax only on the upper surface of the leaflets while other parts were without wax. Thus the waxy1 and waxy2 mutants look very much like the known mutants was and wsp as described in Pisum Newsletter 10:81 (1978).
A study using scanning electron microscopy revealed that on the adaxial side of leaflets of the normal, glaucus line R9, wax was present as a multitude of platelets, 1-2 mm in length, forming a dense cover over the surface (Fig. 1). The same structures, both in shape and number, were also present on the adaxial surface of waxy1 and waxy2 (Fig. 3) leaflets. Crenated ribbons of wax, 4-5 mm in length, were present on the abaxial surface of the leaflets of the normal line R9 (Fig. 2). On the abaxial side of waxy1 leaflets, wax occurred as shorter 2-4 mm ribbons, but mainly as rods and granules (Figs 5 and 6). The greatest reduction in wax occurred with the waxy2 mutant where only very small rods of less than 0.6 mm length and granules were found on the abaxial surface of leaflets (Fig. 4).
The substantial reduction of crystalline wax on waxy2 plants resembles the observation by Holloway et al (2) for the phenotypically identical wsp mutant. So waxy2 and wsp may be identical mutations and the minor differences in details of wax structure may be due to differences to environmental conditions and genetic background.
 |
Fig. 1. Line R9: adaxial side of the leaf; x 10000; the bar above 30* 2 nm = 3 mm. |
|
|
Fig. 2. Line R9: abaxial side of the leaf; x7500. |
|
|
Fig 3. Line waxy2: adaxial side of the leaf; x 10000. |
|
|
Fig. 4. Line waxy2: abaxial side of the leaf; x 5000. |
|
|
Fig. 5. Line waxy1: abaxial side of the leaf; x5000. |
|
|
Fig. 6. Line waxy1: abaxial side of the leaf; x 10000. |
Table 1. F2 segregation data for crosses between waxy1 and marker lines for chromosome 1 loci.
|
Marker gene (X) |
Phase |
Marker lines |
Phenotype |
Chi-square |
Recomb fract |
SE |
||||||
|
Waxy1 |
Waxy1 |
waxy1 |
waxy1 |
Total |
Waxy1 |
X |
Joint |
|||||
|
X |
x |
X |
x |
|||||||||
|
A-a |
Coup. |
Moustached; L63 |
530 |
125 |
125 |
98 |
878 |
0.07 |
0.07 |
54.27*** |
34.0 |
2.1 |
|
Lf-lf |
Coup. |
R1 |
252 |
54 |
46 |
51 |
403 |
0.19 |
0.24 |
46.64*** |
28.9 |
2.8 |
|
Af-af |
Rep. |
Moustached |
369 |
106 |
112 |
30 |
617 |
1.30 |
2.88 |
0.09 |
49.0 |
3.1 |
|
I-i |
Coup. |
Moustached; L63 |
496 |
159 |
178 |
47 |
880 |
0.15 |
1.19 |
1.07 |
52.7 |
2.6 |
***P< 0.001
Crosses of waxy1 and waxy2 plants with each other and with the wb mutant produced only normal plants in F1. Hence the three genes are not allelic. Unfortunately, we had no other known waxless mutants in our collection so a complete set of allelism tests was not possible. Crosses of our mutants with marker lines gave no evidence of linkage between waxy2 and a, lf, af, i, le or r. However, there was strong evidence (P < 0.001) of linkage between waxy1 and chromosome 1 markers a (34 cM) and lf (29 cM) (Table 1). The linkage data with Lf were obtained by crossing with regefterant line R1 which differed from the waxy1 line only at the Waxy1 and Lf loci. So other flowering genes which might hamper an interpretation of cosegregation data were absent in the cross. Our result is in accord with the known value of the A-Lf linkage [about 3 cM by our own studies and about 10 cM from published data (3)]. Therefore we propose the gene sequence A-Lf-Waxy1 in Chromosome 1. Waxy1 did not show linkage with markers af and i in the lower section of chromosome 1 (Table 1). None of the previously described wax loci is on chromosome 1. Hence waxy1 may be a new locus. However, a complete set of allelism tests is necessary to fully resolve the identity of the waxy1 and waxy2 mutants.
Ezhova, T.A., Bagrova, A.M., Hartina, G.A. and Gostimski, S.A. 1989. Genetica (Russ.) 25(5):875-885.
Holloway, P.J., Hunt, G.M., Baker, E.A. and Macey, M.J.K. 1977. Chem. Phys. Lipids 20:141-155.
Murfet, I.C. 1971. Heredity 27:93-110.