|
PNL |
Volume 22 |
1990 |
RESEARCH REPORTS |
pages 55-58 |
PURIFIED GUARD CELL PROTOPLASTS FROM THE LEAF EPIDERMIS OF THE ARGENTEUM MUTANT OF PISUM SATIVUM
|
Smith, S., and J.D-B. Weyers,* P.C. Jewer and H-O. Höglund |
University of Dundee, Dundee, UK University of Leeds, Leeds, UK University of Gothenburg, Gothenburg, Sweden |
*Corresponding author.
The argenteum mutant of Pisum sativum (7,8) has leaves with easily detached epidermis, a property greatly in demand to facilitate studies of stomatal physiology (10). As a result, there have been a number of reports characterizing the in vitro responses of pea stomata which have utilised epidermal strips from this mutant (1,2,6). One of these (6) also described the production of partly purified guard cell protoplasts (GCPs) using arg leaf epidermis as starting material.
GCPs represent a particularly valuable research tool, allowing a range of novel techniques to be applied to investigate the stomatal mechanism (11). However, to allow the full potential of GCPs to be realized, they must be (a) pure and (b) viable. In the former respect, arg poses a problem because of the high number of epidermal cell protoplasts (ECPs) that are normally formed along with the GCPs. This report describes an improved procedure for separating and purifying viable GCPs and ECPs from arg leaf epidermis.
Epidermal strips from arg plants were first plasmolysed, then floated on a digestion medium containing cellulases and a pectinase at 30°C. After 1.5 h, a mixture of ECPs and GCPs was obtained in the ratio 1.7:1 and at yields of 35 and 72%, respectively (these figures being expressed as a proportion of the cells remaining viable after removal of epidermal strips). Very small numbers of mesophyll cell protoplasts (MCPs) were observed: centrifugation of the digestion medium half way through the incubation (3) was not necessary because initial mesophyll contamination in arg epidermal strips was very low (< 0.7 cells mm-2).
The mixed protoplast suspension was washed in the mannitol osmoticum and loaded as the top layer of a three-part discontinuous density gradient based on buffered Percoll media (details in legend to Table 1). ECPs were relatively buoyant and could be harvested 91.0% pure in the 0% Percoll band (yield = 45.4% of those applied to the gradient). At the interface between the 20 and 40% Percoll layers, 97.0% pure GCPs could be obtained at a yield of 35.0% of those applied (Table 1). Importantly, the 3.0% impurity in the GCP suspension was due to ECPs and not MCPs. From peeling to purification, the overall GCP yield was 23%. Thus at the observed abaxial stomatal frequency of 167 pores mm-2 about 130 cm2 of epidermal strips would be required to obtain a pure preparation containing 106 GCPs.
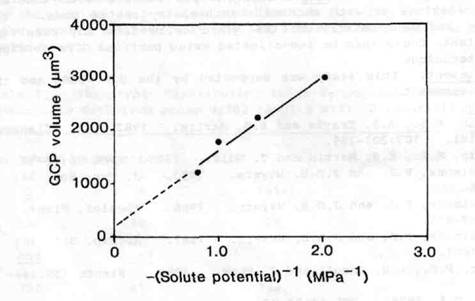
The viability of the purified arg GCPs was tested in two ways. Firstly, the protoplasts were incubated in mannitol media of different concentration. Fig. 1 shows a "Boyle-van't Hoff" plot of protoplast volume against the inverse of the solute potential of the suspension medium. The data exhibit a high degree of linearity, indicating that the GCPs behaved as perfect osometers within the range of solute potentials tested. This can be interpreted as an indication of membrane integrity. The fitted straight line gives rise to estimates of 567 fmol as the mean GCP perfect solute content and 200 mm3 for the mean non-osmotic volume (NOV), values that are small compared with those of other species, probably due to the relatively small size of pea guard cells and the fact that they contain relatively few chloroplasts.
Table 1. Location of protoplasts after centrifugation on a three-part discontinuous density gradient. Abaxial epidermal strips were removed from the 4th and 5th oldest foliage leaves of 6-week-old arg plants grown in peat based compost (seed kindly provided by G.A. Marx). Any strips with visible mesophyll contamination were rejected. The strips were plasmolysed on 400 mol m-3 mannitol for 30 min, then transferred to a digestion medium consisting of 2% (w/v) dialysed Cellulysin (Calbiochem Ltd, La Jolla, USA), 0.0005% (w/v) Pectolyase (Seishin Pharmaceutical Co., Tokyo, Japan), 0.25% (w/v) bovine serum albumin (Fraction V, Sigma Ltd, Poole, UK), 10 mol m-3 MES buffer and 350 mol m-3 mannitol adjusted to pH 5.0 with KOH. The strips were incubated for 1.5 h at 30°C. The resulting protoplasts were harvested and washed by pelleting at 10,000 m s-2 and resuspending in 2 x 10-6 m3 of 350 mol m-3 mannitol. This suspension was loaded as the upper (0% Percoll) phase of the discontinuous density gradient. The middle and lower phases were formed from 2 x 10-6 m3 of 350 mol m-3 mannitol, 10 mol m-3 KCl, 10 mol m-3 MES buffer (pH 7) and Percoll at 20 and 40% (v/v), respectively. The loaded gradient was centrifuged at 10,000 m s-2 for 5 min. Purities are based on at least 10 counts per sampled zone using a haemocytometer.
|
Layer of gradient |
Distribution of cell types* within layer |
||
|
GCPs |
ECPs |
MCPs |
|
|
0% band |
8.5 |
91.0 |
0.5 |
|
0/20% interface |
13.6 |
86.4 |
0.0 |
|
20% band |
82.4 |
17.6 |
0.0 |
|
20/40% interface |
97.0 |
3.0 |
0.0 |
|
40% band |
100.0 |
0.0 |
0.0 |
* Guard cell protoplasts (GCPs), epidermal cell protoplasts (ECPs) and mesophyll cell protoplasts (MCPs).
A second indication of viability came from studies of changes in arg GCP volume induced by two compounds known to affect stomatal movements, namely KCl and the plant hormone abscisic acid (ABA). Table 2 shows the extent of GCP swelling and shrinkage in their presence. The results again indicate a high level of viability, with mean rates of net trans-plasmalemma solute fluxes estimated at 115 nmol (inward) m-2 plasmalemma s-1 (+KCl treatment) and 180 nmol (outward) m-2 plasmalemma s-1 (+KCl, +ABA treatment), assuming all solute gain and loss to be trans-plasmalemma solute transfer at the plasmalemma area at the mid-time of treatment (see 4). These values are similar to those previously determined for Commelina GCPs and to those estimated to occur in and out of guard cells in intact tissues of that species (see 4 and 5). It thus seems that the arg GCPs were not affected adversely by the isolation and purification procedures.
We conclude that the isolation and purification methods described here give rise to very pure arg GCPs of high viability. However, we caution that it might be necessary to adjust the concentrations of Percoll used in the density gradient to suit different batches of plants.
For those wishing to investigate the physiology of pea stomata, the ability to obtain pure GCPs represents an important advance (11). Moreover, at the time of writing, this report increases from two to three the number of species from which large scale preparations of pure GCPs have been obtained (the other two are Commelina communis and Vicia faba). Our procedure can be used to obtain large numbers of partially purified pea ECPs. ECPs are of interest in their own right, because at present they represent the sole way in which these cells can be studied in isolation (3).

Fig. 1. Boyle-van't Hoff plot for purified arg GCPs. GCPs were incubated in 150, 200, 300 or 400 mol m-3 mannitol for 15-30 min. Protoplast volumes were determined from 25 diameter measurements per mannitol concentration. The mean GCP solute content and non-osmotic volume (NOV) were determined by least-squares regression (r2 = 0.95) of the mean protoplast volumes against the reciprocals of the solute potentials of the mannitol media, the estimated NOV being the y-axis intercept of the regression line and the estimated solute content its slope divided by the gas constant and the absolute temperature (see 9).
Table 2. Effect of KCl and ABA on purified arg GCPs. The GCPs were incubated at low CO2 levels under illumination from white fluorescent tubes (about 20 W m-2 PAR) using the system illustrated in Fig. 2a of ref. 9. They were first incubated in 400 mol m-3 mannitol for 15 min, then transferred to this medium plus KCl (at 10 mol m-3) with or without (±)-ABA (at 10 mmol m‑3). Some GCPs were left in the original medium to act as a control. Protoplast volumes were determined from at least 25 diameter measurements per sample.
|
Time (min) |
Treatment |
Mean GCP volume (mm3) |
|
0 |
control |
1770 |
|
36 |
control |
1922 |
|
39 |
+KCl |
2375* |
|
42 |
+KCl, +ABA |
925** |
* Significantly different from 36 min control at P < 0.01.
** Significantly different from 39 min +KC1 treatment at P <
0.001
(Mann-Whitney U-test).
The arg pea mutant continues to prove of value to the stomatal physiologist. We envisage exciting prospects for advances in this field if crosses between arg and other pea mutants with abnormal stomatal behaviour or with abnormal biochemistry can be made. The physiology and biochemistry of the guard cells from any resultant double mutants could then be investigated using purified GCPs obtained with our technique.
Acknowledgement. This study was supported by the U.K. SERC and the Swedish Government.
Donkin, M.E., A.J. Travis and E.S. Martin. 1982. Z. Pflanzen-physiol. 107:201-209.
Donkin, M.E., E.S. Martin and T. Hull. 1983. PNL 15:13-14.
Fitzsimons, P.J. and J.D.B. Weyers. 1983. J. Exp. Bot. 34:55-66.
Fitzsimons, P.J. and J.D.B. Weyers. 1986. Physiol. Plant. 66:469-475.
Fitzsimons, P.J. and J.D.B. Weyers. 1987. J. Exp. Bot. 38:992-1001.
Jewer, P.C., L.D. Incoll and J. Shaw. 1982. Planta 155:146-153.
Marx, G.A. 1978. PNL 10:34-37.
Marx, G.A. 1982. J. Heredity 73:413-420.
Weyers, J.D.B. and P.J. Fitzsimons. 1985. In The Physiological Properties of Plant Protoplasts, Ed. P.E. Pilet, Springer-Verlag, Berlin, pp 152-162.
Weyers, J.D.B. and A.J. Travis. 1981. J. Exp. Bot. 32:837-850.
Weyers, J.D.B., P.J. Fitzsimons, G.M. Mansey and E.S. Martin. 1983. Physiol. Plant. 58:331-339.