CYTOLOGICAL EVIDENCE FOR
BIPARENTAL TRANSMISSION OF PLASTID DNA IN PISUM SATIVUM.
Corriveau, J. L, A. W. Coleman,
Division of Biology & Medicine
Brown University, Providence, RI USA
and N. 0. Polans
Department of Biological Sciences
Northern Illinois University, DeKalb, IL USA
Genetic evidence is available
describing the mode of plastid inheritance for some 60 angiosperm
genera (8,9). In the majority of these genera (including Pisum)
plastids are inherited maternally. As early as 1930, DeHaan (4) reported
that a chlorophyll deficiency was inherited maternally in Pisum
sativum. Since then, to our knowledge, DeHaan's observations have
been neither corroborated nor challenged.
DNA-fluorochromes are being used
increasingly in pollen biology (2,6). Recently, our lab reported a
DNA-fluorochrome/epifluorescence microscopy protocol which permits
rapid screening for plant species potentially capable of biparental
transmission of plastid DNA (1). When pollen was examined from plant
species known genetically to display biparental plastid transmission, e.g.
Oenothera biennis and Pelargonium zonale (9), plastid DNA
aggregates (plastid nucleoids) were detected in the cytoplasm of the
generative and/or sperm cells. However, in species known genetically
to display strictly maternal
transmission, e.g. Mirabilis jalapa and Nicotiana tabacum
(9), no plastid nucleoids were observed.
The purpose of the present study
is to determine if the cytological evidence for the mode of plastid DNA
transmission in Pisum sativum corroborates the earlier genetic report.
Mature pollen grains obtained
from greenhouse-grown pea plants were subjected to cytologica] analysis as
described by Coleman and Goff (2). Living pollen grains were t irst Incubated at
20-23C for 3 h in depression wells containing 0.5 ml germination medium
(20% sucrose plus 0.01% H3BO3 and 0.02%
CaCl2 in distilled water). Germinated pollen was then fixed in
95% ethanol:glacial acetic acid (3:1) overnight at 4C, before being
transferred to 70% ethanol for storage at 4C. Samples were prepared by
allowing a drop of fixed
pollen to dry on a slide followed by staining with 0.05 mkg/ml
4',6-diamidino-2-phenylindole (DAPI) in McIlvaine's buffer (pH 4). Observations
of DAPI-DNA fluorescence were made using a Zeiss AXI0PH0T epifluorescence microscope equipped
with a 50 W mercury lamp and the Zeiss 48-77-02 combination of excitation
and emission filters. DNase-treated controls served to monitor the
specificity of staining for
DNA. Pea plants were scored as potentially capable of biparental transmission of plastid DNA if plastid nucleoids were
observed in the cytoplasm of the generative cells of germinated pollen.
They were scored as presumably maternal if no plastid nucleoids were
observed. At least 100 pollen grains were examined for each pea
line.
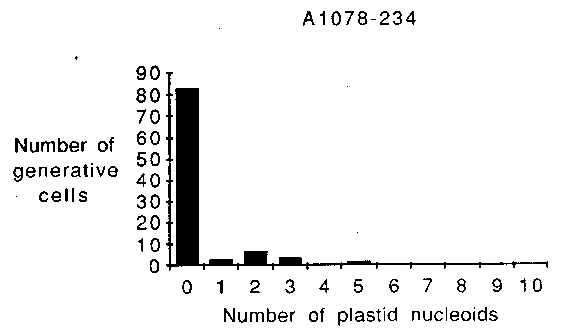
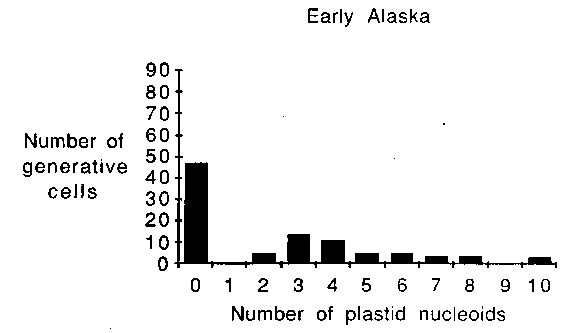
Cytological evidence obtained
from eight pea accessions and cultivar Alaska (Table 1) suggests that
plastid DNA can be transmitted biparentally in P. sativum.
Plastid nucleoids were observed in the generative cells of germinated
pollen grains from each of the pea lines examined. Variability was
observed, however, among these lines with regard to both the
percentage of pollen grains potentially capable of transmitting
plastid DNA (Table 1), and the number of plastid nucleoids found per
generative cell