18
PNL Volume 19 1987 RESEARCH REPORTS
SOMATIC EMBRYOGENESIS IN PEA
Kysely, W. and H.-J. Jacobsen Institute of Genetics
University of Bonn, D-5300 Bonn 1
Federal Republic of
Germany
Whole plant regeneration via somatic embryogenesis was
ob-
tained in pea using explants from immature embryos or shoot
apex
segments. Explants were placed on medium supplemented with MS
salts
(3), B5 vitamins (1), 3% sucrose, and 0.7% Phytagar (Gibco).
Among auxin
treatments, picloram and 2,4-D at 4.0 mkM in the ab-
sence of any
cytokinin were most efficient for producing somatic
embryos. After 25 to 35
days in culture, somatic embryos of dif-
ferent sizes could be observed on
explants from immature embryos
and shoot apex segments (Fig. 1,2). The level
of auxin necessary
for the induction of somatic embryos apparently prevented
further
development of young embryo stages and also repressed the
germina-
tion of fully-developed embryos. Consequently, somatic
embryos
were transferred to a medium with only cytokinin (1.0 mg/l BAP)
or
with cytokinin in combination with a reduced auxin concentration
(0.05
mg/1 NAA and 0.017 each of BAP, kinetin, and zeatin) (Fig.
3). Somatic
embryos were obtained from immature zygotic embryos 2
to 9 mm in length. More
data are presented in a paper published
elsewhere (2).
Generally somatic embryos arise from the callus derived
from
embryogenic axes of zygotic embryos but can also develop
directly
from the cotyledon without an intervening callus phase.
Somatic
embryos from shoot apex cultures obviously emerge from the
main
and axillary shoot meristem regions.
Figs. 4a,b show longitudinal sections of a mature
somatic
embryo with a well-defined shoot meristem with leaf
primordia
(Fig. 4a) and a root meristem (Fig. 4b). The meristems are
con-
nected by procambium strands.
The results obtained so far indicate that there are
genotypic
differences in frequency of somatic embryogenesis from
immature
embryos and shoot apices, thus indicating that the ability to
form
somatic embryos is a quantitative rather than a qualitative
trait.
1. Gamborg, 0. L., R. A.
Miller, and K. Ojima. 1968. Exp.
Cell Res. 50:151-158.
2. Kysely, W., J. R. Myers,
P. A. Lazzeri, G. B. Collins, and
H.-J. Jacobsen. Plant Cell Rep.
(submitted).
3. Murashige, T. and F.
Skoog. 1962. Physiol. Plant. 15:
473-479.
PNL Volume 19 1987 RESEARCH REPORTS 19
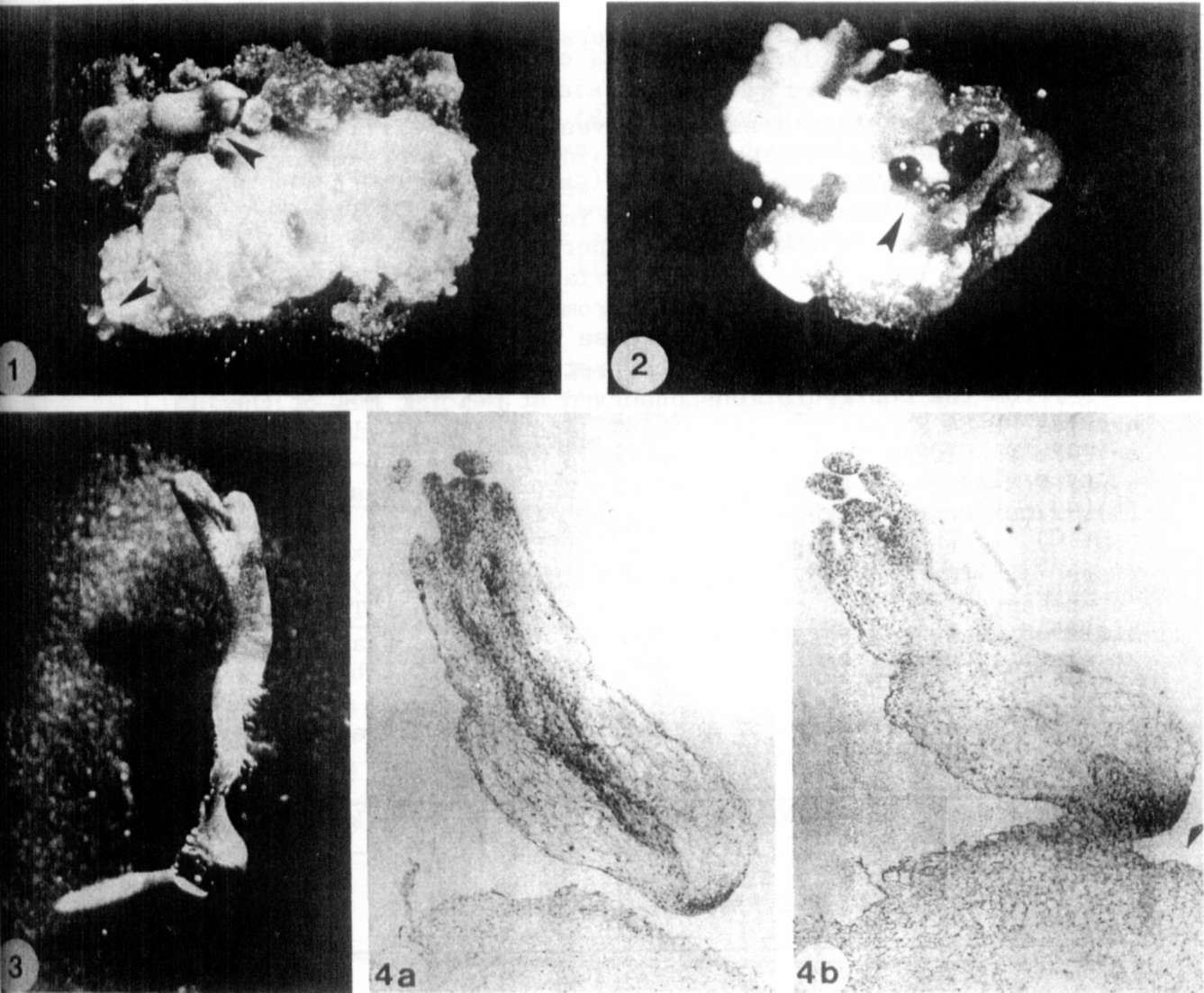
Fig.1. Somatic embryos from immature embryo culture
of
genotype R 4111, induced on medium with
4.0 mkM
Picloram.
Fig.2.Somatic embryos from a shoot apex culture of
genotype
P. sativum var. arvense, induced on medium with
4.0
mkM Picloram.
Fig.3. A germinating somatic embryo of P. sativum var.
arvense on medium with 1.0 mg/l BAP.
Fig.4. Longitudinal sections of a mature
somatic
embryo of P_. sativum var. arvense, induced
on
medium with 0.2 mkM Picloram. Sections were stained with
Haeomatoxylin.
a Section demonstrates the shoot meristem with leaf
primordia and procambium strands,
b Section showing the root meristem.
* * * * *