PNL Volume 17 1985
RESEARCH REPORTS 7 7
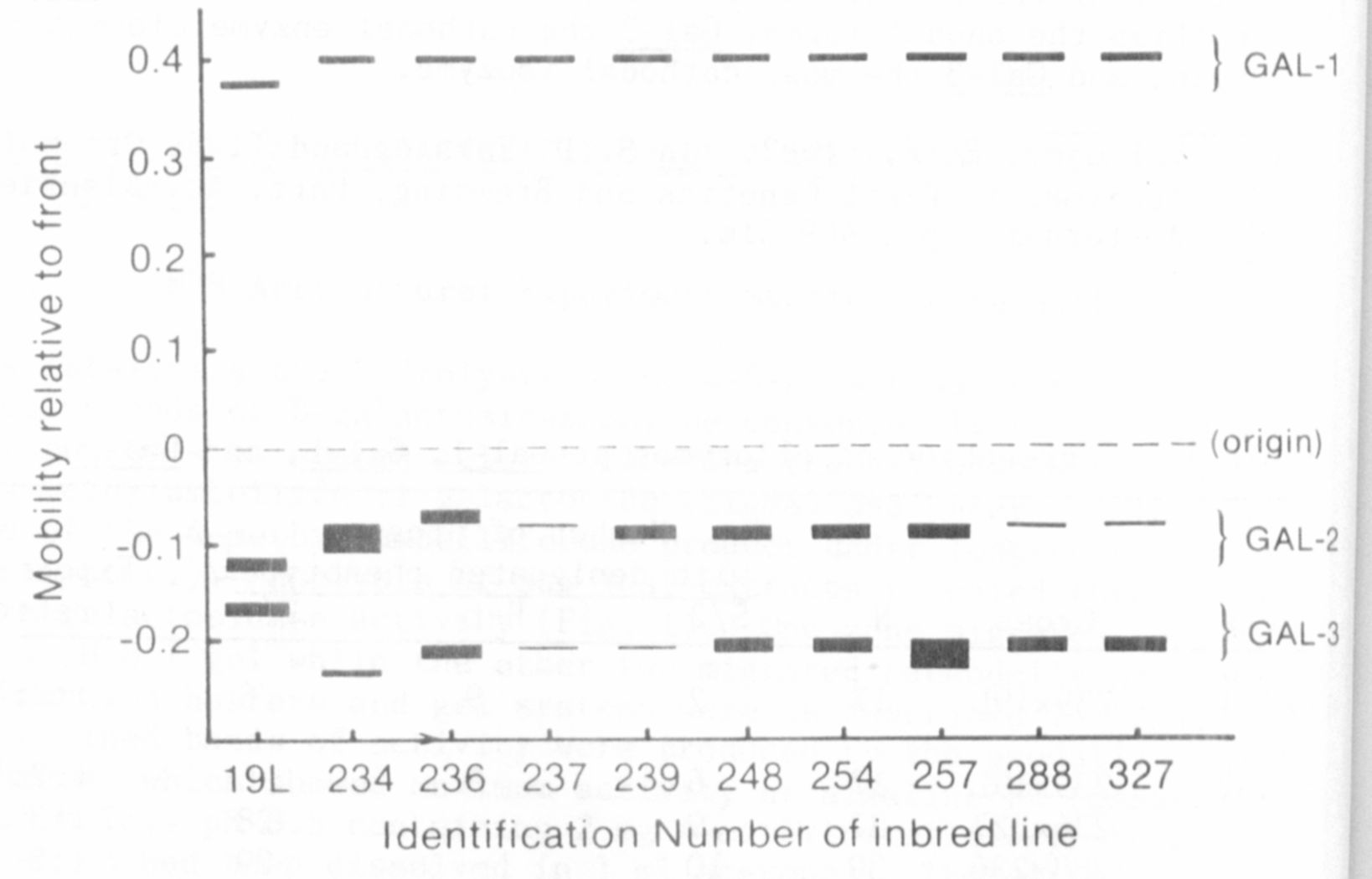
A survey of inbred lines provided by Dr. G. A. Marx and several
P.I. accessions revealed polymorphism for all three zones of beta-galac-
tosidase activity. Variants of the anodal enzyme were rare and identi-
fied by a different electrophoretic mobility. In contrast, variants in
the cathodal regions were relatively common and often exhibited a dif-
ference in level of activity (Fig. 1).
In order to determine if the three zones of activity represented
products of different loci, appropriate crosses were made and the F2
populations analyzed for segregation within each zone. Certain of the
variants showed so little activity that they were treated as "null"
alleles. In these cases the expected ratio was 3:1, for the heterozy-
gotes were difficult to distinguish from the homozygous "active" pheno-
type and thus were grouped together as "galactosidase plus". For cases
in which the mobility of the variants differed, three classes of progeny
could be distinguished, and the expected ratio was 1:2:1.
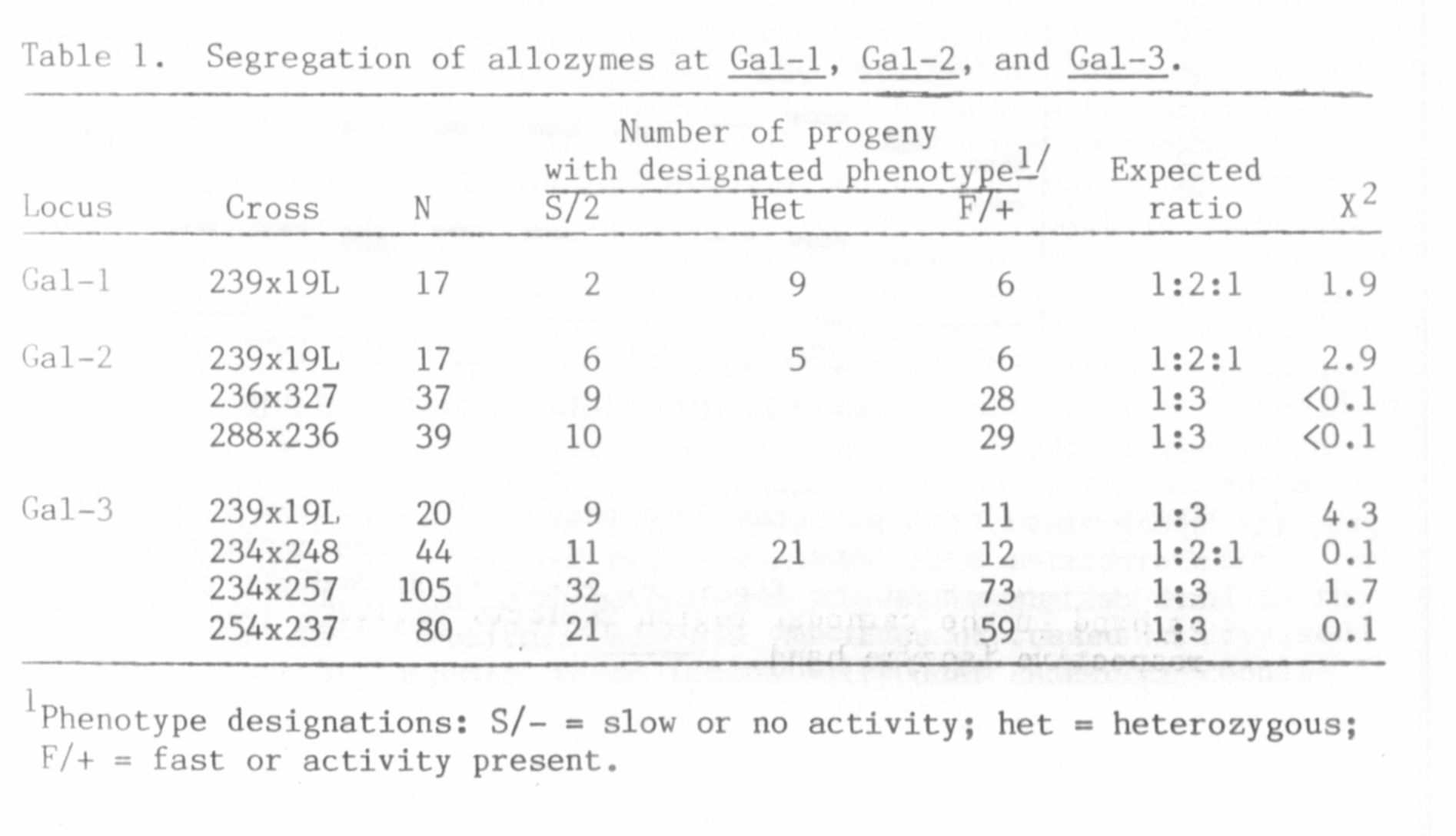
The segregation observed within individual zones is summarized in
Table 1. The variants within a zone appeared to be products of dif-
ferent alleles, for normal segregation ratios were obtained in all
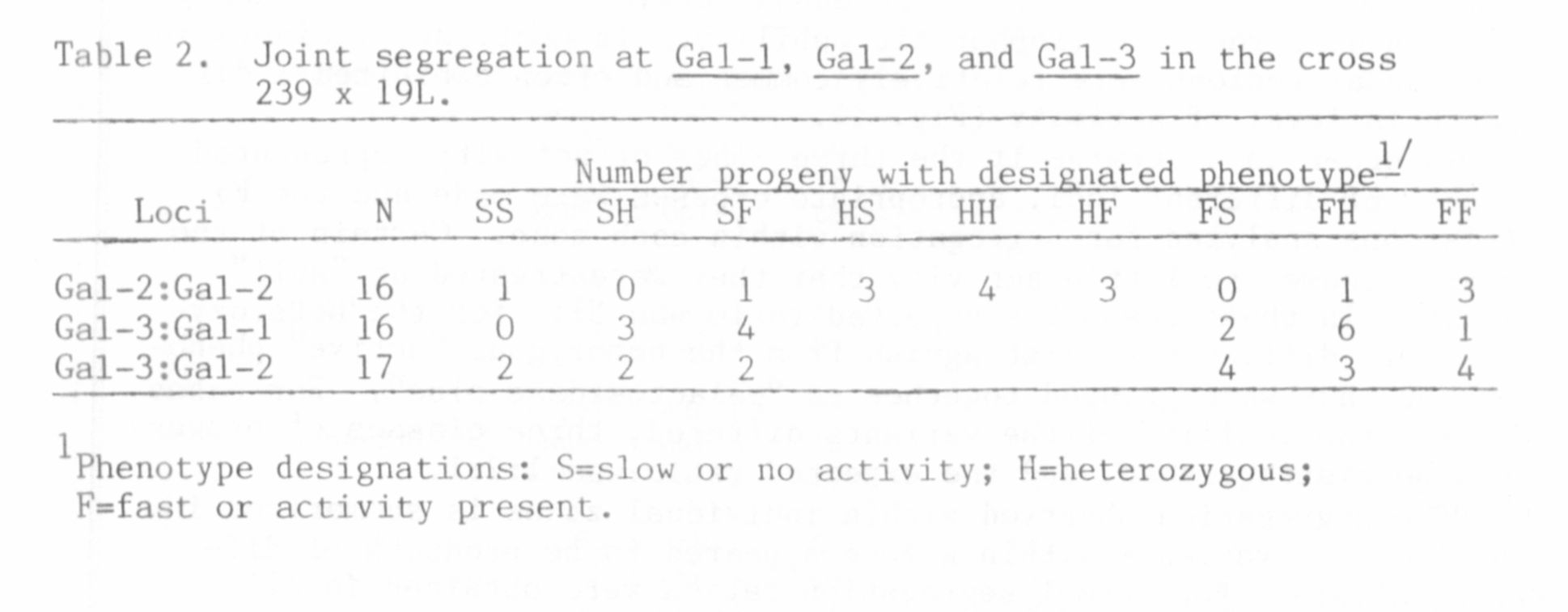
cases. That each zone of activity represented a distinct locus was
demonstrated by joint segregation analysis presented in Table 2.
Although the size of the populations listed in Table 2 was too small to
eliminate the possibility of loose linkage between loci, the significant
level of recombination exhibited between each pair of loci establishes
the presence of three distinct beta-galactosidase loci in pea. The loci
have been designated Gal-1, Gal-2, and Gal-3 in order of relative
mobility of their isozymes in the anodal direction. Thus Gal-1
specifies the anodal forms, Gal-2 the cathodal enzyme closest to the
origin, and Gal-3 the most cathodal isozyme.
1. Vallejos, E. E. 1983. In S. D. Taksley and T. J. Orton (eds.).
Isozymes in Plant Genetics and Breeding, Part. A. Elsevier,
Amsterdam, pp. 469-516.