PEA ENATION MOSAIC VIRUS: VARIATION IN RESISTANCE CONFERRED BY En
Baggett, J. R. and R. 0. Hampton Oregon State University
Corvallis, Oregon
Resistance to pea enation mosaic was reported by Schroeder and
Barton (2) to be conferred by a single dominant gene, En. They noted
that when resistant pea plants were inoculated with any of several pea
enation mosaic virus (PEMV) isolates, the plants usually became in-
fected, with only minor effects on growth. Hagedorn and Hampton (1)
demonstrated that commercial breeding lines putatively containing En
varied in degrees of PEMV-susceptibility, as indicated by symptom
severity. Even the most resistant lines, when inoculated in greenhouse
tests, either developed mild symptoms or, if affected by the virus more
severely, progressively recovered from symptoms. Follow-up field tests
confirmed that lines able to recover from PEM symptoms in the greenhouse
also manifested field resistance, corroborating the presence of the En
allele.
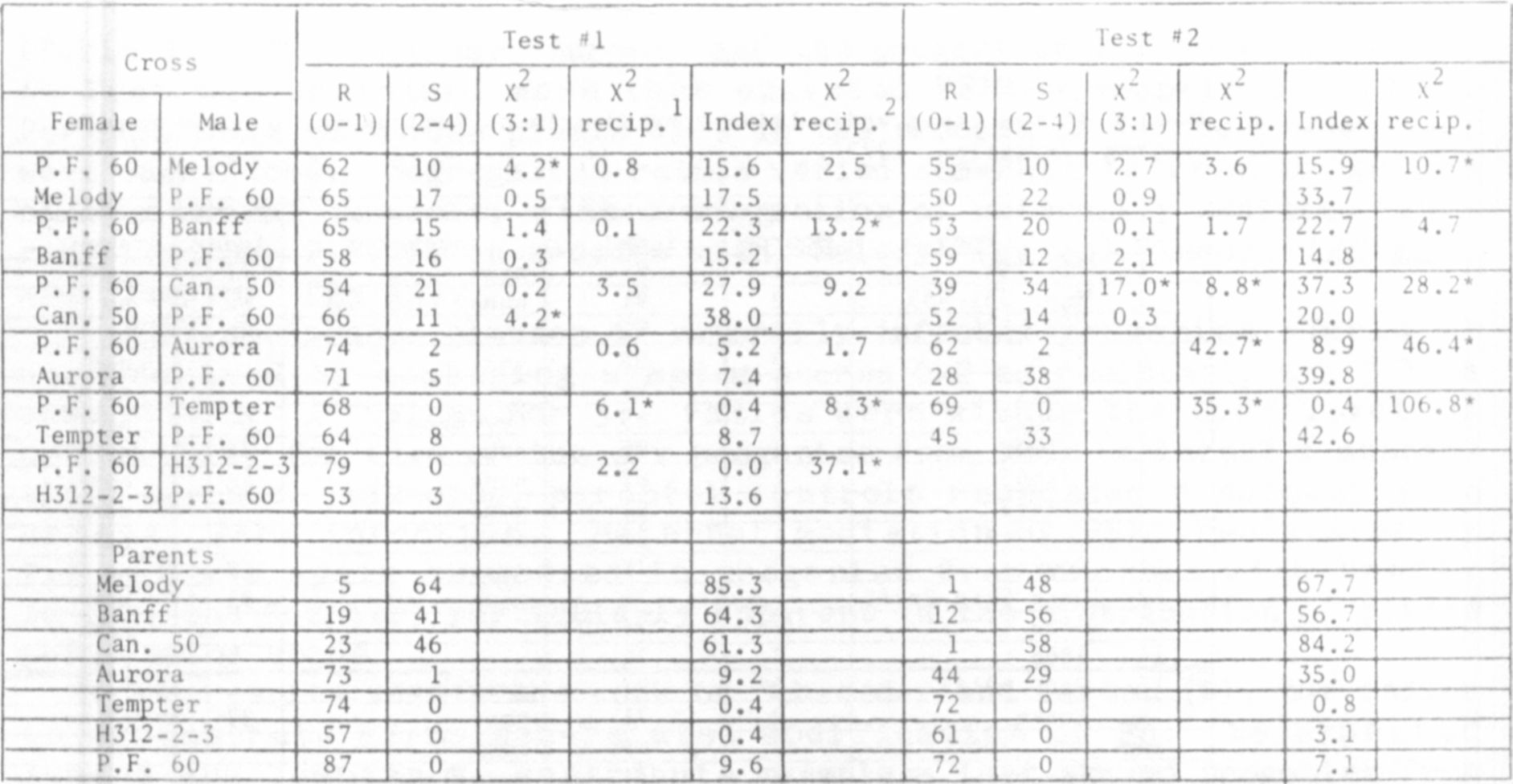
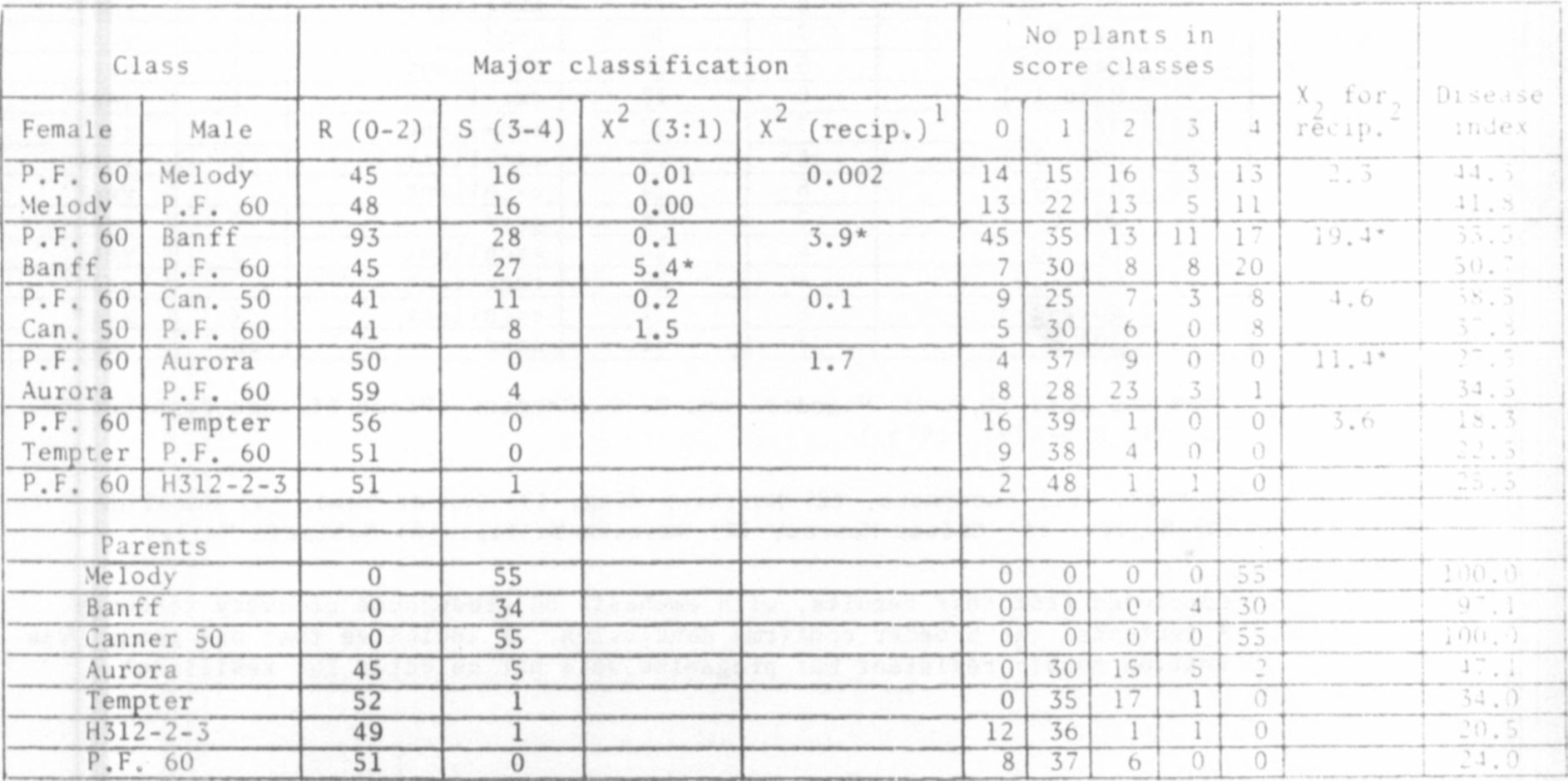
The data of Hagedorn and Hampton have been retabulated in descend-
ing order of susceptibility (disease indices), from greenhouse tests
(Table 1). To these data we have added a column to indicate presumptive
presence of the En allele, as deduced from greenhouse recovery data and
information provided by the breeder of the cultivar. The 45 entries in
Table 1 comprise a continuous range of PEMV responses including interm-
ediate types which, based on PFM- recovery, contain either En or en
alleles (see Line Nos. 15-25). These data suggsst that modifier genes
and perhaps cytoplasmic factors, working either independently of or
interactively with gene En, affect the type and severity of PEM
symptoms. Thus, among these intermediate lines, some with en showed a
lower disease index than some lines carrying En.
We sought to elucidate the nature of this modification by crossing
'Perfected Freezer 60' (En En) reciprocally with three susceptible
(en en) and three other resistant cultivars. F2 progenies from these
crosses were rub-inoculated in two successive "greenhouse tests, using
mechanically transmissible Wisconsin isolate (C3) of PEMV used by
Hagedorn and Hampton (1). These progenies were also field tested
Corvallis under a severe natural incidence of PEMV. Disease indices
were calculated from individual-plant symptom scores, where
visible symptoms, 1 = slight chlorotic flecking, 2 = moderate PEM
symptoms, 3 = severe PEM symptoms, and 4 = PEMV-induced plant necrosis.
To obtain a refined estimate of disease severity, we removed the p1ants
from containers at peak development of PEM symptoms and made side-by-
side visual comparison. This procedure, however, wa3 done at the
expense of greenhouse PEM-recovery data.
Symptom scores of greenhouse-grown progenies from PEMV-resistant x
susceptible parents were combined into resistant (scores 0 and 1) and
susceptible (scores 2-4) classes for computation of segregation ratios.
Most X values for 3:1 ratios were within a 0.05 level of probability,
clearly indicating conformity with and affirming single-dominant-gene
inheritance for PEMV resistance (Table 2). There were a few non-
conforming ratios. For example, control plants of 'Banff and
'Canner PL' tended to escape PEMV-infection during greenhouse inoculation,
particularly in Test #1, but were extremely susceptible in field tests