REGENERATION OF GENE LINES 0F PISUM SATIVUM FROM CALLUS CULTURES
Malmberg, R- L. Michigan State University, East Lansing, MI, U.S.A.
This study was begun with the hypothesis that the ability to regenerate
plants from somatic cell cultures of Pisum might have a genetic basis, and
further that the requisite alleles which allow regeneration would be more
likely to be found in the more primitive pea lines. This hypothesis has support
in other crop species; certain less selected varieties of maize (1) and tomato
(2) have been found to show an increased ability to regenerate from callus
over the currently used crop varieties. On this basis, the standard Pisum
varieties 'Frosty' and 'Alaska' were compared for the ability to regenerate from
callus culture with 14 lines obtained from the collection of G. A. Marx, Geneva,
N.Y.
Seeds of 16 lines were surface sterilized by soaking in 10% chlorox and
0.1% sodium dodecyl sulfate for 5 minutes, rinsing with sterile distilled
water, dipping in 95% ethanol, and then rinsing again in sterile distilled
water. The seeds were placed on Murashige and Skoog (3) medium with no hormones
and solidified with 0.9% agar. After 2 to 3 days incubation in the dark at
26°C, the radicle emerged. As soon as this was observed, the seeds were
dissected and the embryo was removed. Cylindrical sections of the epicotyl
approximately 2 mm in length were placed on a callus inducing medium consisting
of Murashige and Skoog salts and vitamins plus 2 mg of naphthalene acetic
acid and 1 mg of benzyladenine per liter of medium. Sections of epicotyl
were also placed immediately on regeneration medium consisting of the same
salts and vitamins but with 0.2 mg of indoleacetic acid and 5 mg of benzyl-
adenine per liter. Callus plates were kept in the dark at 26°C, and regenera-
tion plates were kept at room temperature under a cool white fluorescent light
of intensity approximately 2500 lux under a 16 hour/8 hour light/dark regime.
The epicotyl sections of different lines gave rise to callus at different
rates, but all lines grew, approximately doubling in every month. The callus
was transferred to fresh callus medium every month, and every other month
a portion was subcultured and placed on regeneration medium. Thus each line
was tested for regeneration from 0 months callus (epicotyl), 2 months growth
as callus, 4 months growth, and 6 months (most recent test). On the regen-
eration medium, the greenest, most organized structures were subcultured
monthly onto fresh regeneration medium until well defined shoots with leaf
nodes formed. When they occurred, typically 2 to 3 months after initiation
of regeneration, these well formed shoots were rooted by slicing them off
just below a leaf node; the lowest leaves were removed, and then the shoot
bottom was dipped in sterile naphthalene acetic acid, 1 mg per ml, to a level
just above the bottom node. The shoot was planted in medium without hormones
with the shoot bottom immersed in the agar to a level just above the lowest
leaf node, and then kept at room temperature under light as in shoot regenera-
tion. After approximately a month, when roots had emerged from the buried
node, the plantlets were transferred to sterile soil, after the agar had been
gently washed away. The plantlets were gradually brought to greenhouse condi-
tions with special care at initially keeping the humidity very high.
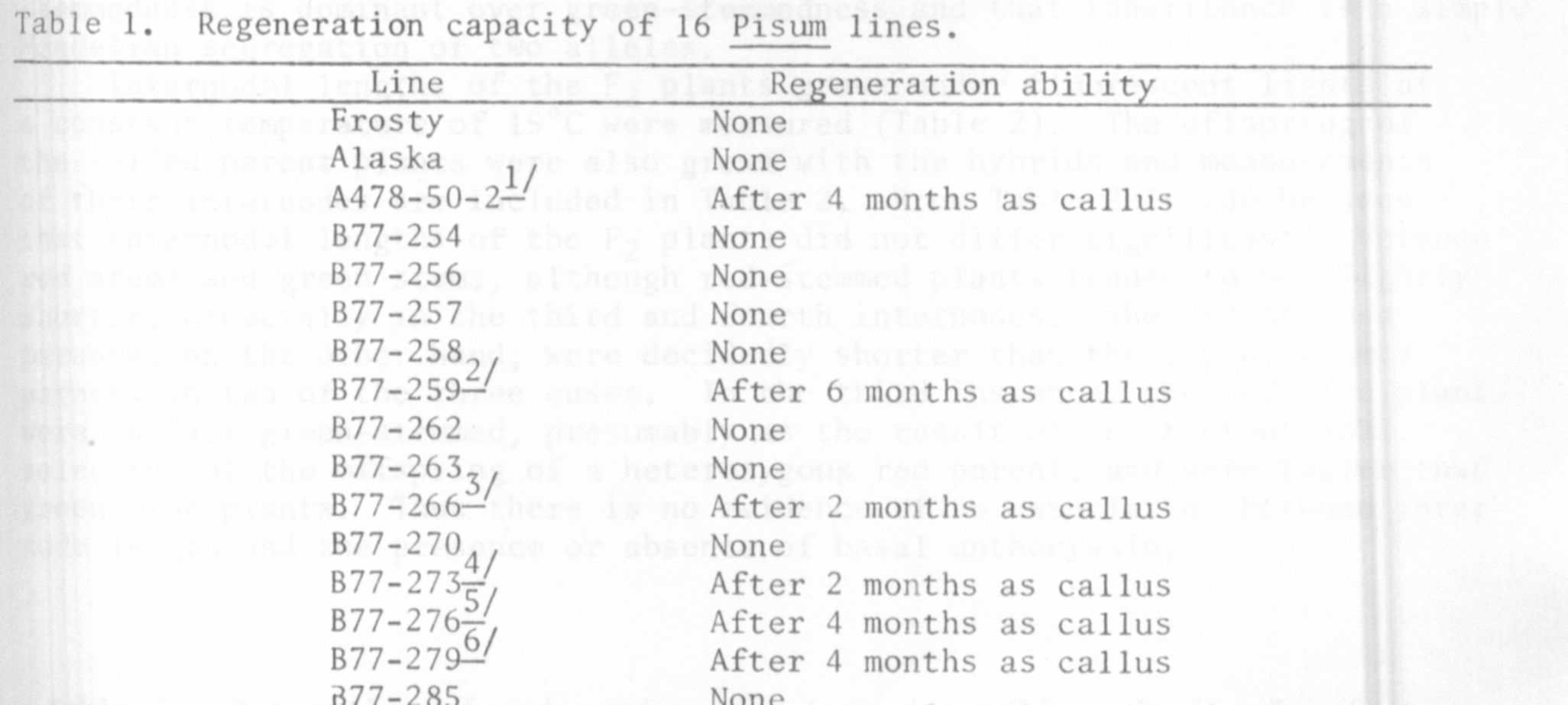
Table 1 shows the results of this comparative study of regeneration among
pea lines used. Listed are the various lines tested, and the last month
of callus culture from which they were able to regenerate. Six were able