RLSHARCH REPORTS
PNL Volume 11
1979
complex is eluted for 45 mm in test tubes containing 5 ml 0.1% sodium dodecyl
sulfate (SOS) solution. Then the solution is decanted into cuvettes and the
absorbance is read at 600 nm with a Beckman spectrophotometer against the eluate
of the blank set at 0.000 absorbance.
To determine the value of this assay in Pisum sativum seed protein, extrac-
tions, different amounts of seed flour of our initial line ('Dippes gelbe
Viktoria'J, were dissolved in a KCl-buffer (0.2 mol KC1, pH 6.85) to give an
estimated final concentration from 0.5 to 2.5 mg protein per ml. When the
extraction process lasts up to 48 hours, uniform and reproducible results were
obtained. Six replicates of each extraction were measured and compared with
a BSA regression line (Fig. 1) established by concentrations reaching from
0.5 to 2.5 mg protein per ml. These results are in good agreement with those
obtained by the Kjeldahl method. So far BSA seems to be a useful standard
tor estimating the seed protein values in Pisum until purified Pi sum seed
protein is available.
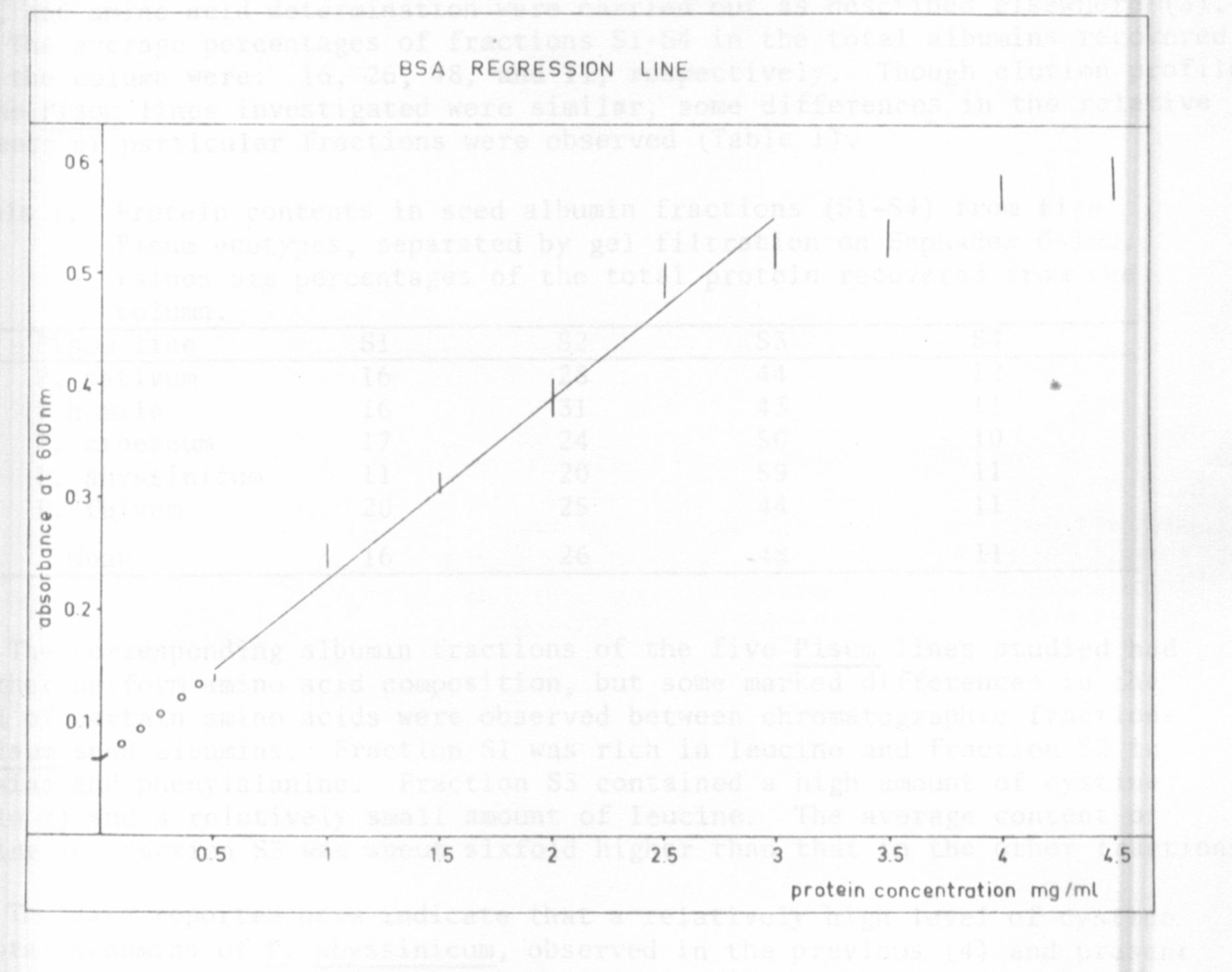
Fig. I. BSA-regression line established by concentrations reaching from
0.5 to 2.5 mg protein per ml.